Abstract
The use of electronic cigarettes, or “vaping,” has garnered significant popularity and attention in recent years. Its pulmonary and systemic effects have yet to be fully studied and quantified, and recent reports of vaping-related illnesses and deaths have brought the clinical consequences of vaping into the public spotlight. This report describes the case of a 34 year old woman who presented to clinic with new-onset cough and dyspnea, shortly after beginning to use electronic cigarettes. Imaging demonstrated new micronodular opacities and mediastinal lymphadenopathy, while pathology confirmed granulomatous disease. After she received counseling and successfully quit vaping, her symptoms resolved and repeat imaging demonstrated resolution of parenchymal findings and lymphadenopathy. This case report therefore presents a longitudinal narrative of reversible vaping-related pulmonary granulomatous disease.
1. Text
1.1. Case description
A 34 year old woman presented to clinic with dry cough and dyspnea. She was a former cigarette smoker (four pack-years, quit at age 32). Her medical history was significant for a low-grade carcinoid tumor, which was discovered the year prior when she had sought evaluation for recurrent pneumonia and wheezing. At that time, due to airway obstruction, she had undergone right middle and lower lobectomies, and her symptoms resolved shortly thereafter.
During the current presentation, one year post-bilobectomy, she reported new-onset dry cough, dyspnea and wheezing. She did not experience fevers, weight loss, hemoptysis, sick contacts, or overt occupational exposures. A computed tomography (CT) scan revealed new multifocial clusters of micronodules bilaterally with enlarged mediastinal and hilar lymph nodes.
Empiric antibiotic treatment (amoxicillin-clavulanic acid) for infectious bronchiolitis and community-acquired pneumonia failed to resolve her symptoms; instead, a repeat CT scan one month later demonstrated worsening reticulonodular opacities and mediastinal lymphadenopathy (Fig. 1, Fig. 2)
Fig. 1.
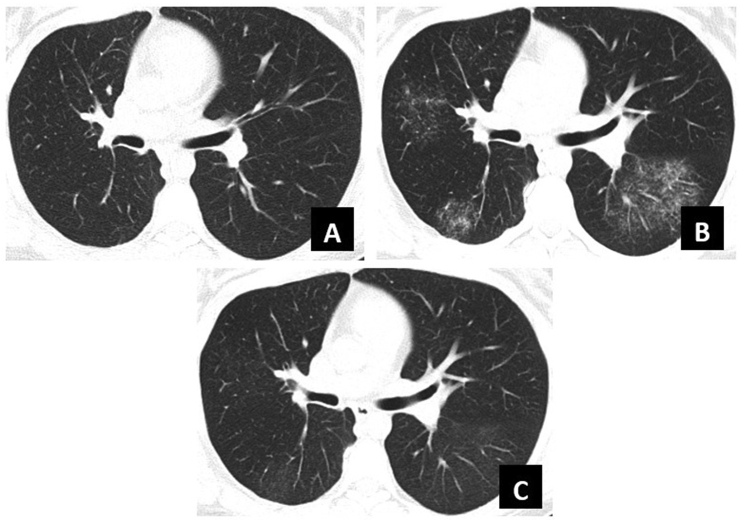
Chronologic progression of CT scan findings
A: Surveillance CT scan, six months after bilobectomy for carcinoid tumor.
B: Repeat CT scan, one month after symptom onset and three months after vaping initiation.
C: Follow-up CT scan, six months after vaping cessation and symptom resolution.
Fig. 2.
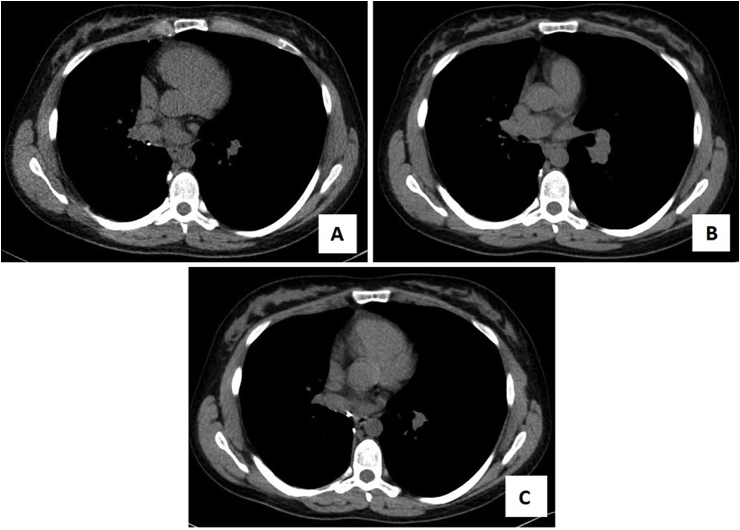
Chronologic progression of CT scan findings, mediastinal (Width 350, Level 50) windows.
A: Surveillance CT scan, six months after bilobectomy for carcinoid tumor.
B: Repeat CT scan, one month after symptom onset and three months after vaping initiation.
C: Follow-up CT scan, six months after vaping cessation and symptom resolution.
Careful history elicitation retroactively revealed that she had started inhaling heated aerosols from electronic cigarettes (“vaping”) approximately two months prior to the onset of symptoms. She had been vaping on a daily basis, using a Vuse Alto® device, without simultaneous cannabinoid use or systemic corticosteroid use.
She underwent bronchoscopy with bronchoalveolar lavage, revealing clear and colorless supernatant. Bacterial, fungal and mycobacterial cultures were negative. Cell count revealed 148 nucleated cells/L, predominantly macrophages (87%) with few neutrophils (5%) and lymphocytes (8%). CD4:CD8 ratio was 1.00, below the laboratory threshold consistent with suspected sarcoidosis. Endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes showed benign lymphoid cells and non-necrotizing granulomas in the right paratracheal, subcarinal, and left hilar lymph node stations. A transbronchial biopsy of the left lower lobe demonstrated foci of non-necrotizing granuloma, rare histiocytic clusters with multinucleated giant cells and scattered eosinophils (Fig. 3). Concurrent serum values did not reveal leukopenia nor eosinophilia; erythrocyte sedimentation rate was unremarkable. Alkaline phosphatase, transaminases, electrolytes, and serum albumin were all within normal ranges.
Fig. 3.
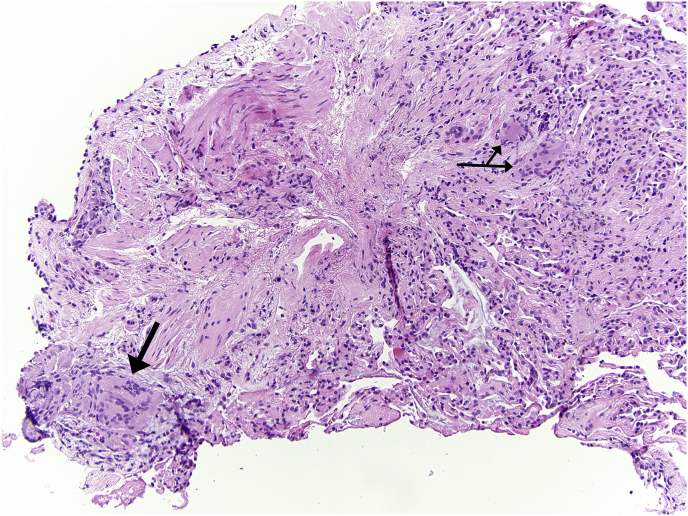
Left lower lobe transbronchial biopsy: minute foci of non-necrotizing granulomas (arrows) with multinucleated giant cells.
The clinical diagnosis was therefore pulmonary granulomatous disease related to vaping. The patient received counseling, and with the use of nicotine replacement therapy, she successfully quit vaping without complication. Follow-up CT scan after six months of abstinence revealed resolution of the lung opacities, as well as thoracic lymphadenopathy (Fig. 1). Her symptoms resolved and she did not require additional treatment.
1.2. Discussion
This case of pulmonary granulomatous disease related to vaping describes unintended consequences from vaping. Vaping simulates the experience of traditional cigarette smoking by vaporizing liquids which are then inhaled.1 Despite a relative lack of evidence-based literature, many consider vaping to be lower risk when compared to traditional cigarette smoking, presumably due to the absence of conventionally-visualized and combustion-produced toxicants, and their purported role in assisting with traditional smoking cessation.[1], [2], [3]
In the United States, more than three million people are estimated to be routine participants in vaping.4 Prevalence of vaping has dramatically increased in the last several years, most notably among the adolescent population.5 Among growing populations, Hispanic youth are excessively affected as the highest proportion of users.1 While only 1.3% of adults over the age of 45 who vape were active or prior cigarette smokers, approximately 40% of those between 18 and 24 years who vape had never smoked cigarettes, suggesting a possible “gateway” effect of vaping.6
Aerosols that are inhaled from vaping contain numerous compounds, including nicotine, metal nanoparticles, flavoring compounds, and toxins such as formaldehyde and acetaldehyde.7,8 While alveolar absorption of nicotine is akin to that in traditional cigarette smoking, airway injury and absorption of other components is not as well-understood.
Cases of pulmonary disease and inhalation injuries related to vaping abound: the Centers for Disease Control and Prevention has identified over 450 such cases in the summer of 2019 alone, and the first of several vaping-related fatalities was reported in August.9 An “Investigation Notice” was posted this same month regarding these cases, for which the underlying commonality is an antecedent history of vaping.10
This case report describes reversible pulmonary granulomatous disease and reaffirms an association between vaping and lung injury, evidenced clinically and radiographically. Moreover, it characterizes a chronological history of granuloma development after vaping initiation, followed by resolution upon cessation. This patient's symptoms are additionally consistent with a sarcoidosis-like reaction, but in the absence of corroborative serum or clinical history, a diagnosis of sarcoidosis was felt to be less likely. While pulmonary granulomatous disease has been well-studied in cases of chronic silica and asbestos exposure, it appears that vaping-related granulomatous disease may be a separate, reversible entity.11,12 Similar reports would justify studying the association between vaping initiation and development of pulmonary granulomatous disease.
Given the increasing popularity of vaping, further research can assist with determination of toxic doses and guidance for regulations regarding safe levels of components within liquids for vaping. Importantly, such investigation can bolster public knowledge with objective evidence that vaping is not benign, and has known pulmonary consequences. Electronic cigarette manufacturers could accurately report health risks associated with use of their products, and marketed items could be considered for mandated labeling in a manner similar to that of the Surgeon General's Warning located on traditional cigarettes.
Declaration of competing interest
No conflicts of interest exist for the following authors: Charlie Lin, Valeria Arrossi, Ruchi Yadav, Humberto Choi.
Contributor Information
Charlie Lin, Email: [email protected].
Valeria Arrossi, Email: [email protected].
Ruchi Yadav, Email: [email protected].
Humberto Choi, Email: [email protected].
References
- 1.Jenssen B.P., Walley S.C. Section on tobacco Control. E-Cigarettes and similar devices. Pediatrics. 2019;143(2) doi: 10.1542/peds.2018-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zulkifli A., Abidin E.Z., Abidin N.Z. Electronic cigarettes: a systematic review of available studies on health risk assessment. Rev. Environ. Health. 2018;33(1):43–52. doi: 10.1515/reveh-2015-0075. [DOI] [PubMed] [Google Scholar]
- 3.St Helen G., Eaton D.L. Public health consequences of e-cigarette use. JAMA Intern Med. 2018;178(7):984–986. doi: 10.1001/jamainternmed.2018.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serror K., Chaouat M., Legrand M.M. Burns caused by electronic vaping devices (e-cigarettes): a new classification proposal based on mechanisms. Burns. 2018;44(3):544–548. doi: 10.1016/j.burns.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Gentzke A.S., Creamer M., Cullen K.A. Vital signs: tobacco product use among middle and high school students - United States, 2011-2018. MMWR Morb. Mortal. Wkly. Rep. 2019;68(6):157–164. doi: 10.15585/mmwr.mm6806e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Electronic cigarettes. What's the bottom line? https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/Electronic-Cigarettes-Infographic-p.pdf. Accessed September 3, 2019.
- 7.Thirion-Romero I., Perez-Padilla R., Zabert G., Barrientos-Gutierrez I. Respiratory impact of electronic cigarettes and "Low-Risk" tobacco. Rev. Invest. Clin. 2019;71(1):17–27. doi: 10.24875/RIC.18002616. [DOI] [PubMed] [Google Scholar]
- 8.Kaur G., Pinkston R., McLemore B., Dorsey W.C., Batra S. Immunological and toxicological risk assessment of e-cigarettes. Eur. Respir. Rev. 2018;27(147) doi: 10.1183/16000617.0119-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richtel M., Kaplan S. First death in a spate of vaping sicknesses reported by health officials. The New York Times. August 23, 2019 https://www.nytimes.com/2019/08/23/health/vaping-death-cdc.html [Google Scholar]
- 10.Centers for Disease Control and Prevention. Outbreak of severe pulmonary disease associated with using e-cigarette products. US Dept of Health and Human Services. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Accessed September 3, 2019.
- 11.Pulmonary granulomatosis. J. Am. Med. Assoc. 1965;193(4):302. [Google Scholar]
- 12.Nevins M.A., Stechel G.H., Fishman S.I., Schwartz G., Allen A.C. Pulmonary granulomatosis. Two cases associated with inhalation of cosmetic aerosols. J. Am. Med. Assoc. 1965;193(4):266–271. doi: 10.1001/jama.1965.03090040010002. [DOI] [PubMed] [Google Scholar]