Introduction
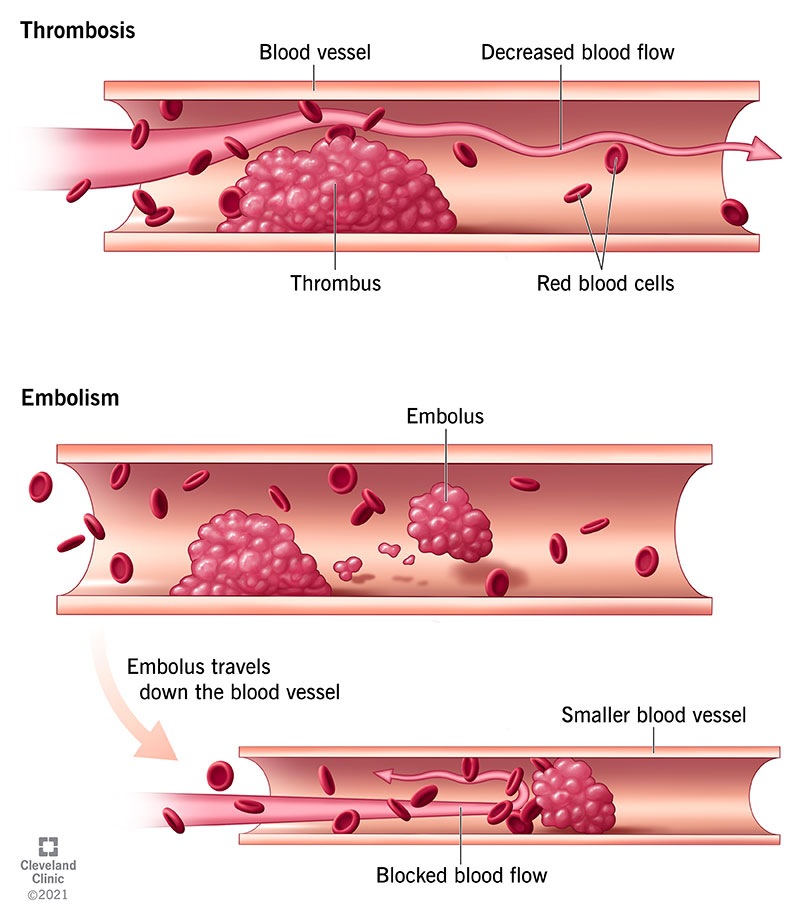
Temporomandibular joint (TMJ) ankylosis is a condition that can affect all age groups and is observed throughout the world. The primary effect of ankylosis is severe restriction of mandibular range of motion, which may limit nutritional intake, interfere with hygiene, limit evaluation and treatment of oral cavity disease, and perilously limit airway access. Kazanjian classified TMJ ankylosis as true or intra-articular and false or extra-articular depending on whether the pathology lies inside or outside the joint (1). TMJ ankylosis is usually referred to as either bony or fibrous. In the case of fibrous ankylosis, dysfunction arises from tenacious fibrous soft tissue growing between two closely-apposed bony surfaces (Figure 1). In these cases, range of motion may be somewhat less limited than in bony ankylosis. In contrast, with bony ankylosis, there is fusion of the condylar head to the base of skull, usually in the glenoid fossa and temporal bone area (Figures 2,3). Mandibular range of opening is typically extremely limited, and what is present may be simply from flexion of the mandible without any actual motion within the joint.
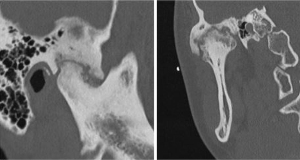
Figure 1 Fibrous ankylosis on CT scan in sagittal and coronal planes.
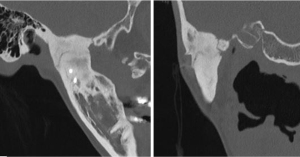
Figure 2 Bony ankylosis on CT scan in sagittal and coronal planes. This patient had a prior costochondral graft.
Figure 3 Bony ankylosis in two patients with no visible demarcation between the temporal bone and the condyle.
Ankylosis of the TMJ most commonly arises from direct trauma or infection (either regional, such as mastoiditis or an ear infection, or septic arthritis) although many other conditions may contribute to ankylosis. These include ankylosing spondylitis, rheumatoid arthritis, reactive arthritis, prior surgical intervention with exuberant heterotopic ossification, congenital deformities, and idiopathic factors (2).
Treatments for TMJ ankylosis have evolved over several decades and vary regionally. Most commonly practiced around the world in adults is a gap arthroplasty, with or without interpositional tissue (i.e., a temporalis myofascial flap, silastic block, or adipose tissue). Other treatments have been described such as distraction osteogenesis and joint reconstruction using autogenous grafts—including costochondral grafts (CCG) and clavicular bone grafts—or alloplastic prostheses (3).
In many areas of the world, ankylosis is treated with a total TMJ prosthesis (total joint reconstruction or TJR) (2,4-6). This has gained popularity due to the lack of requirement for donor site for reconstruction as well as the predictable successful outcomes in terms of restoration of range of motion and infrequent postoperative reankylosis. While there are many case series and other studies evaluating other methods of management of TMJ ankylosis and there are many studies confirming the success of modern prosthetic total joint arthroplasty, there is less data specifically evaluating prosthetic reconstruction with a total joint prosthesis in these challenging cases. The primary purpose of this article is to review the published scientific literature to answer the question, “In non-growing patients with TMJ ankylosis, what are the outcomes when treated with a total prosthetic joint reconstruction?” A secondary purpose of this study was to evaluate outcomes for patients with TMJ ankylosis treated with TJR at the authors’ institution. The hypothesis of the study is that treatment of TMJ ankylosis with TJR is an effective and safe means of improving range of motion and minimizing recurrence. The article is presented in accordance with the PRISMA-ScR reporting checklist (available at https://fomm.amegroups.com/article/view/10.21037/fomm-22-15/rc).
Methods
This is both a retrospective cohort study of Mayo Clinic patients and a review of the literature. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Mayo Clinic Institutional Review Board (No. 21-001585) and individual consent for this retrospective analysis was waived.
Data sources and search strategies
A comprehensive search of several databases was performed on January 18, 2022. Date limits were set from 1990 forward. Animal studies were excluded. No language restrictions were applied. Databases searched were Ovid MEDLINE(R) 1946 to Present and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid Embase 1988+, Ovid Cochrane Central Register of Controlled Trials 1991+, Ovid Cochrane Database of Systematic Reviews 2005+, Web of Science 1975+, and Scopus 1788+. Figure 4 is a flow diagram illustrating the screening process for evidence used.
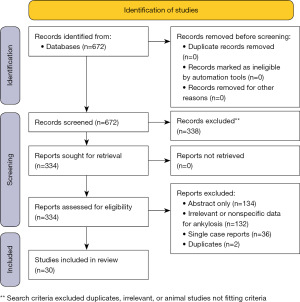
Figure 4 PRISMA 2020 flow diagram.
The search strategy was designed and conducted by an experienced librarian with input from the study’s investigators. Controlled vocabulary supplemented with keywords was used to search for studies describing alloplastic joint reconstruction for TMJ ankylosis. The actual strategy listing all search terms used and how they are combined is available in Appendix 1. The scoping review protocol is registered on the Open Science Framework at https://doi.org/10.17605/OSF.IO/M6NKZ (7).
In addition to these search methods, references within relevant scholarly articles were also cross-referenced to verify inclusion of all applicable studies. Inclusion for this study required treatment of ankylosis with a total joint prosthesis and data including specific outcomes for treatment of TMJ ankylosis, including maximum incisal opening (MIO) and complications such as reankylosis. Single case reports and patients still undergoing jaw growth were excluded, as were studies where there were mixed treatment modalities or mixed diagnoses and separation of data was not clear.
Review of all studies was undertaken by two authors (AA and WJF) to confirm inclusion was appropriate. Any disagreements would have been resolved by an appeal to the third author. The data was charted in a secure spreadsheet, and included: author, country, year of publication, study design, age group, follow up duration, number of all cases, number of ankylosis cases who underwent total joint replacement, etiology of ankylosis, previous TMJ surgeries, preoperative and postoperative MIO for the ankylosis cases, type and design of the prosthesis, one- or two-stage surgery, concomitant surgeries (i.e., orthognathic procedures), operative complications, and reankylosis.
Results of the studies were compiled for evaluation (Table 1). Primary outcomes were range of motion MIO before and after reconstruction and complications of treatment. Secondary variables measured included etiology, prior TMJ surgeries, type of prosthesis, change in MIO, and reankylosis.
Table 1
Included studies
| Author and year | Study type | All cases (n) | Ankylosis cases (n) | Mean age (years) | Etiology [n] | Mean follow-up [range], months | Preop MIO, mm | Postop MIO (mm) | Change in MIO (mm) | Implant Type | Complications [n] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wolford et al., 2016 (5) | Retrospective cohort | 32 | 32 | 39 | Trauma [17]; chronic arthritis [2]; osteochondroma [1]; syndromes [3]; ankylosing spondylitis [1]; failed previous reconstruction [1]; unknown [3]; TMJ disorder [1]; iatrogenic [3] | 59.5 [12–168] | 14.5 [6.3–20] | 35 [30–40] | 20.5 | TMJ Concepts | Heterotopic bone [2]; hardware loosening in syndromic patient [1]; infection [1]; reankylosis [1]* |
| Egemen et al., 2012 (6) | Retrospective cohort | 5 | 5 | 24 | Trauma [4]; congenital [1] | 11.2 [6–21] | 5.2 [3–8] | 33.2 [29–38] | 28 | Biomet (stock) | Intraoperative bleeding [1]; temporary CN VII weakness [2]; permanent facial nerve weakness [1] |
| Alessandra et al., 2012 (8) | Retrospective cohort | 9 | 9 | Male: 42.3; female: 45.5 | Trauma [9] | 24–96 | 6.6 [5.3–11] | 32 [27–37] | 25.4 | Biomet (stock) | Infection [1]; salivary fistula [2] |
| Amarista et al., 2022 (9) | Retrospective cohort | 28 | 28 | 42 | DJD [10]; trauma [8]; DJD and osteoarthritis [4]; JIA [2]; unknown etiology [1] | 46 [6–101] | 16.9 | 28.77 | 11.87 | TMJ Concepts | Prosthetic infection [2]; limited MIO requiring brisement [2]; unsightly scar [1] |
| Balon et al., 2019 (10) | Retrospective cohort | 12 | 4 | 48.25 | Trauma [4] | 24–67 | 16.75 [2–30] | 38.5 [30–45] | 21.75 | Biomet (stock) | Temporary CN VII weakness [4]; hypoesthesia [3] |
| Bhardwaj et al., 2016 (11) | Retrospective cohort | 22 | 5 TJR | 25.4 | Trauma [22] | 24–96 | 2.8 [1–5] | 43.6 [42–46] | 40.8 | NM | Intraoperative bleeding [1] |
| †Bhargava et al., 2020 (12) | Prospective cohort | 20 | 18 | 28.75 | NM | 6–12 | 3.1 | 33.01 [29–38] | 29.91 | DARSN TM Joint Prosthesis (custom) | None |
| Brabyn et al., 2019 (13) | Retrospective cohort | 6 | 4 | 47.25 | NM | >6 | 13 [0–20] | 30.5 [24–34] | 17.5 | Custom CAD/CAM prosthesis | Temporary CN VII weakness [1] |
| ‡Chowdhury et al., 2019 (14) | Retrospective cohort | 8 | 8 | 27.5 | Trauma [7]; infection [1] | 12 | 2 [0–7] | 31.8 [28–36] | 29.8 | Biomet (Stock) | Occlusal discrepancy [2]; temporary CN VII weakness [1] |
| Gerbino et al., 2016 (15) | Retrospective cohort | 12 | 12 | 44.3 | Trauma [3]; congenital and infection [9] | 49 [18–150] | 7.9 [1–20] | 27.3 [18–40] | 19.4 | Biomet (6 custom, 6 stock) | Temporary CN VII weakness [4]; intraoperative bleeding [1]; occlusal discrepancy [3] |
| Gruber et al., 2015 (16) | Prospective cohort | 58 | 6 | 47 | NM | 12–36 | 10.5 | 31.3 | 20.8 | TMJ Concepts | NM |
| §Gundlach et al., 2010 (17) | Retrospective cohort | 6 | 5 | 32 | Trauma [5]; radiation [1] | 160 [115–235] | 8 [2–15] | 19 [15–23] | 11 | Stainless steel fossa and condyle | NM |
| Haq et al., 2014 (18) | Retrospective cohort | 5 | 5 | 44.6 | Rheumatoid arthritis [2]; degenerative changes [1]; juvenile idiopathic arthritis [1]; infection [1] | 19 [9–27] | 1.4 [0–5] | 25 [23–27] | 23.6 | Biomet (custom) | Heterotopic bone [1] |
| Hu et al., 2017 (19) | Retrospective cohort | 11 | 11 | 45 | Trauma [8]; infection [3] | 21 [12–31] | 5.5 [0–18] | 31.6 [25–35] | 26.1 | Biomet (stock) | None |
| Jones, 2011 (20) | Prospective cohort | 7 | 2 | 55.7 | NM | 12–36 | 5 [2–8] | 31.5 [28–35] | 26.5 | Mixed (TMJ Concepts and Biomet stock) | None |
| Kim et al., 2021 (21) | Retrospective cohort | 8 | 2 | 58.8 | Trauma [2] | 36 | 31 [19–43] | 42 [44–40] | 11 | Biomet (stock) | None |
| Kunjur et al., 2016 (22) | Unclear | 15 | 4 | 33.5 | NM | 30 [18–48] | 2.75 [0–10] | 25.75 [23–30] | 23 | Biomet (custom) | NM |
| Linsen et al., 2013 (23) | Prospective cohort | 17 | 8 | 47 | Trauma [3]; rheumatoid arthritis [2]; degenerative joint disease [1]; radiotherapy [1]; septic arthritis [1] | 22.9 [12–51] | 18.57 [5–30] | 30.50 [17–42] | 11.93 | Mixed (TMJ Concepts and Biomet stock) | NM |
| ¶Loveless et al., 2010 (24) | Retrospective cohort | 36 | 36 (14 TJR) | 40 | Systemic [3]; Iatrogenic [5]; other [6] | 12 [0.3–105] | 15.6 | 24.9 | 9.3 | Mixed (TMJ Concepts, Biomet, Christensen) | NM |
| Machon et al., 2012 (25) | Retrospective cohort | 27 | 12 | Male: 35.4; female: 42.6 | NM | 48 | 10.2 [3–20] | 29.75 [25–48] | 19.55 | Biomet (stock) | NM |
| Mani et al., 2020 (26) | Retrospective cohort | 10 | 10 | 17.8 | Trauma [10] | 48 | 5.70 | 34.9 | 29.2 | Custom | None |
| Mercuri et al., 2008 (27) | Retrospective cohort with survey | 20 | 20 | 44 | Trauma [11]; DJD [3]; rheumatoid arthritis [2]; facial burn [1]; multiple prior surgeries [3] | 50.4 [24 –108] | 11.75 [0–25] | 32.9 [20–42] | 21.15 | TMJ Concepts | NM |
| Pearce et al., 2009 (28) | Retrospective cohort | 5 | 5 | 40.6 | Trauma [3]; condylar hypoplasia [1]; hemifacial microsomia [1] | 9.4 [4–16] | 7.4 [5–12] | 25.4 [22–33] | 18 | TMJ Concepts | Neuropathic pain [1]; temporary CN V paresthesia [2] |
| Rikhotso et al., 2021 (29) | Retrospective cohort | 31 | 18 | 30 | NM | 58.8 [6–122] | 1.83 | 25.53 | 23.7 | Biomet (stock and custom) | Heterotopic bone [2] |
| ‡Roy Chowdhury et al., 2019 (30) | Retrospective cohort | 12 | 12 | 34 | NM | 12 | 3.92 [1–7] | 32.67 [28–36] | 28.75 | Biomet (stock) | Occlusal discrepancy [2]; infection [1]; intraoral ramal component exposure [1]; pain and reduced mouth opening [1] |
| Roychowdhury et al., 2021 (31) | Prospective cohort | 41 | 41 | 25.12 | Trauma [30]; infection [7]; ankylosing spondylitis [1]; unknown [3] | 31.73 [12–65] | 5.17 | 35.32 | 30.15 | Biomet (stock) | Occlusal discrepancy [8]; CN VII weakness [9]; dislocation of condyle [1] |
| ¶Sahdev et al., 2019 (32) | Retrospective cohort | 95 | 42 | 44.3 | NM | 53.76 [6–86.76] | 18.9 | 29.3 | 10.4 | TMJ Concepts | NM |
| Westermark et al., 2010 (33) | Retrospective cohort | 12 | 5 | 29 | Trauma [2] | 60 [24–96] | 3.8 | 30.2 | 26.4 | Biomet (stock) | NM |
| Yadav et al., 2021 (34) | Retrospective cohort | 114 | 41 (TJR) | 15.75 | Trauma [87]; infection [20]; others [ankylosing spondylitis, idiopathic] for all cases | 25.98 [12–80] | 5.17 | 35.32 | 30.15 | NM | NM |
| Zou et al., 2018 (35) | Retrospective cohort | 33 | 10 | 51.5 | NM | 21.48 [12–77] | 8.63 | 32.88 | 24.25 | Biomet (stock) | NM |
†, this study included 20 patients, 18 of whom had ankylosis, but individual data for ankylosis was not included. We decided to include this despite lack of ankylosis-specific data; ‡, these articles may present some of the same subjects, as the two studies are from the same author over the same time period; §, this study used a stainless steel prosthesis with nonstandard techniques and fixation; ¶, these articles may have some overlap of subjects, as they are retrospective studies of similar patient criteria from the same institution in overlapping time periods; *, reankylosis in this case developed around the coronoid, and not necessarily the TJR prosthesis itself. TMJ, temporomandibular joint; TJR, total joint reconstruction; NM, not mentioned; MIO, maximum incisal opening; DJD, degenerative joint disease; JIA, juvenile idiopathic arthritis; CAD/CAM, computer-aided design/computer-aided manufacturing; CN, cranial nerve.
For the retrospective cohort study, the Mayo Clinic medical records from 2014–2021 were reviewed for cases that met the same inclusion criteria as the literature review. 10 cases were identified, and descriptive statistics were tabulated.
Results
Literature review
A total of 30 studies met inclusion criteria from the search results of 672 studies (Table 1). In these studies, 717 cases with the age range of 15 to 80 years were noted. However, only 398 were ankylosis cases that underwent alloplastic total joint replacement. Only studies where separate, clear data for ankylosis patients were included, albeit with some important exceptions noted in the table. The subjects of the study population (n=398) had a minimum follow up duration of 6 months and maximum of 235 months. Etiology of TMJ ankylosis was identified in 20 studies, and included trauma (n=235), infection(n=41), degenerative changes (n=15), rheumatoid arthritis (n=6), juvenile idiopathic arthritis (n=3), and others (n=55, including osteoarthritis, ankylosing spondylitis, congenital, prior TMJ surgeries, radiation, septic arthritis, iatrogenic, facial burn, osteochondroma, condylar hypoplasia, hemifacial microsomia and idiopathic. The other 10 studies did not discuss etiology.
A detailed overview of the preoperative and postoperative MIO is shown in Table 1. The reported MIO data obtained were limited for ankylosis cases undergoing alloplastic total joint replacement. When evaluating mean MIO, the study values varied widely for both preoperative (range, 1.4–18.9 mm) and postoperative (19 to 43.6 mm). Change in mean MIO likewise varied, ranging from 9.3 to 40.8 mm.
Eighteen studies utilized Zimmer Biomet stock and custom implants, 9 studies utilized custom-made TMJ Concepts (Ventura, CA; now Stryker/TMJ Concepts, Ventura, CA) prostheses, and 4 studies utilized customized or stock prostheses of other manufactures. Nineteen studies reported a total of 233 adverse events specifically for ankylosis subjects. These included temporary facial paresis (n=23), occlusal discrepancy (n=15), heterotropic ossification (n=5), permanent facial nerve weakness (n=4), and others (n=24, including infection, salivary fistula, intraoperative bleeding, scar revision, intraoral ramal component exposure, pain and reduced mouth opening, dislocation of condyle, reankylosis, and postoperative neuropathic pain). 23 studies reported no recurrence of ankylosis, while one study reported one case of reankylosis, and 6 studies did not discuss reankylosis.
Case series
Review of the Mayo Clinic patient records yielded sufficient data to include 10 patients undergoing prosthetic joint replacement for treatment of ankylosis. Mean post-surgical follow-up was 13.3 months (range, 3–36 months). Demographic statistics are summarized in Table 2. Mean age at treatment was 41 years (range, 18–72 years), with 2 (20%) being male. Cause of ankylosis is summarized in Table 2, with the most common being prior open TMJ surgery (discectomy, which was performed in 30% of cases). Other case characteristics are summarized in Table 2. Seven (70%) cases had previously undergone open TMJ surgery, and 4 (40%) cases were bilateral reconstructions. All cases used abdominal fat grafting as part of the surgery.
Table 2
Mayo Clinic cohort
| Study variables | Descriptive statistics |
|---|---|
| Sample size (n) | 10 |
| Female | 8 (80%) |
| Age, mean [range], years | 41 [18–72] |
| Unilateral TJR | 6 (60%) |
| Previous open surgeries | 1.2 [0–2] |
| Etiology | |
| Previous open TMJ surgery (discectomy) | 3 (30%) |
| Trauma | 1 (10%) |
| Idiopathic | 1 (10%) |
| Juvenile idiopathic arthritis | 1 (10%) |
| Ankylosing spondylitis | 1 (10%) |
| Multiple distraction osteogenesis | 1 (10%) |
| Extended IMF (13 weeks) | 1 (10%) |
| Ablative surgery with radiotherapy | 1 (10%) |
| MIO (mm) | |
| Preoperative MIO, mean [range] | 11.5 [0–22] |
| Postoperative MIO, mean [range] | 33.5 [20–48] |
| Change in MIO, mean | 22 |
| Type of prosthesis | |
| Custom prosthesis | 9 (90%) |
| One or two-stage procedure | |
| Two-stage | 7 (70%) |
| Complication | |
| None | 6 (60%) |
| Transient facial nerve weakness | 3 (30%) |
| Heterotropic bone formation | 1 (10%) |
| Re-ankylosis | 0 (0%) |
TJR, total joint reconstruction; TMJ, temporomandibular joint; IMF, intermaxillary fixation; MIO, maximum incisal opening.
Mean preoperative and postoperative MIO were 11.7 and 33.5 mm, respectively, with a mean change in preoperative to postoperative MIO was 22 mm. A total of 14 joint prostheses were implanted, with 12 (85.7%) of these being TMJ Concepts patient-fitted prosthesis, and 2 joints (14.3%) being Zimmer Biomet stock prostheses. Regarding complications, three patients had transient facial nerve weakness and one had heterotropic ossification which required debridement and fat grafting (Table 2). None of these patients experienced reankylosis.
Discussion
The dual purpose of this study is to first summarize the scientific literature regarding the use of alloplastic total joint arthroplasty specifically for treatment of TMJ ankylosis and then report the safety and efficacy of this treatment in the Mayo Clinic patient population. We hypothesized that this treatment in cases of ankylosis is both safe and effective and has predictably low morbidity with good results in terms of improved range of motion and low rates of reankylosis.
The published literature summarized in Table 1 appears to confirm the hypothesis, consistently showing a durable improvement in range of motion over a reasonable time period (almost always more than 1 year, and much longer in many instances) for both fibrous and bony ankylosis cases. The studies reviewed showed that adverse events were generally few, and, aside from rare prosthetic joint infection, events were the same as those that are well established risks with any TMJ arthroplasty for treatment of ankylosis (bleeding, facial nerve weakness, malocclusion, etc.) (3,36,37). Permanent facial nerve weakness is a feared complication of any open TMJ surgery, and can result in dry eye, difficulty expressing oneself, and other functional and esthetic challenges (38). Saeed and McLeod retrospectively evaluated trends in facial nerve injury after TJR in a review of 133 cases, noting a 28% incidence of weakness at 2 weeks, but only a 3% rate of permanent weakness (39). They found that permanent weakness was difficult to predict, but bilateral surgery, revision TJR, prior TMJ surgeries, diagnosis leading to surgery, and prior history of recovered facial nerve injury all increased the likelihood of a temporary weakness. Failed TJR had the highest risk of temporary palsy, but no other single diagnosis (DJD, ankylosis, arthropathy, pathology, or trauma) appeared to represent an independent risk factor for weakness in this group. Heterotopic ossification was reported in 5 cases, while reankylosis was reported in only 1 case. Not all studies reported complications, so there is a possibility these may be underreported. Fortunately, many of these complications are managed well intraoperatively or are usually temporary (facial nerve weakness and malocclusion) and do not require additional surgical management.
In the Mayo Clinic case series, outcomes of TJR were very favorable, with improved range of motion (mean improvement was 22 mm), and few serious adverse events. One patient with JIA developed heterotopic bone around the joint prosthesis (albeit without reankylosis) that required removal. One patient with multiple prior open TMJ surgeries suffered a long-term facial nerve weakness. Patients generally experienced good functional outcomes, achieving the desired outcome of the surgery, and with minimal adverse outcomes.
Regarding reankylosis or heterotopic ossification, fat grafting is often employed to minimize this risk. Our own case series involved only patients who had received a fat graft. In addition, two cited studies explicitly discuss fat grafting and its benefits and the conceptual mechanism of action (5,27). Among the studies reviewed, fat grafting was infrequently mentioned, and there are no non-fat control groups within the studies. There is a comparison study by Wolford (40), although this does not explicitly involve ankylotic patients. It does appear that fat grafting is a helpful adjunct, but the studies reviewed do not address it sufficiently to include in our analysis.
There are previous literature reviews regarding TMJ ankylosis treatment. Mittal et al. (3) conducted a systematic review of surgical management of TMJ ankylosis in 2018 that reviewed gap arthroplasty, interpositional gap arthroplasty, reconstruction arthroplasty, and distraction osteogenesis as treatment modalities for this patient population. Interestingly, alloplastic materials used seemed to have a higher rate of reankylosis in their study, but this was more with interpositional alloplastic materials than with an alloplastic TJR. Those with alloplastic reconstruction showing recurrence came from a single cited paper (11) where the alloplastic materials used were a titanium reconstruction plate with a condylar head. This is not comparable to the type of arthroplasty presented in this paper. Mittal et al. assert that “only a smaller number of comparative studies could be included to justify the success of total joint replacement by alloplastic implants.” There is some merit to this, as the literature is inconsistent in terms of the types of prostheses used and the reporting of their use specifically in ankylosis cases. Still, it seems unfair to conclude that they are not justified in their use when basing data off of reports of prostheses that are not FDA-approved devices we recommend to our patients.
Al-Moraissi et al. (36) also addressed TMJ ankylosis surgery three years earlier in a systematic review and meta-analysis. They evaluated mostly comparative studies, and alloplastic reconstructions were compared to CCG in this study using three published studies. They found MIO improvement to be greater in CCG than prosthetic joint reconstruction, whereas CCG was inferior in terms of pain reduction. Saeed et al. (37) and Tang et al. (41) aboth showed no recurrence in alloplastic reconstruction as compared to reankylosis rates of 37% and 18% in the CCG population. Interestingly, the post-ablative prostheses used in Tang et al. did not include a fossa component, but placed a metal condyle against the bony fossa. In addition, the Saeed et al. paper reported on Christensen alloplastic joints, which we do not feel are an accurate surrogate for more modern prostheses currently available for use (they have been removed from the market by the FDA due to prosthetic failures and complications). The paper by Loveless et al. (24) includes some Christensen prostheses but also FDA-approved ones, such as Biomet and TMJ Concepts devices. However, the 9.4 mm gain in MIO seems quite different from this study across multiple papers and in the Mayo Clinic experience, where the mean improvement was 22 mm.
We should also note that in the Mayo Clinic cohort, prior discectomy seemed to be a more common etiologic factor, or at least an antecedent to the need for TJR, occurring in 30% of cases. A prior study on the outcomes of discectomy and fat graft indicated that progressive pain and degenerative changes post-discectomy were observed in nearly 25% of subjects, while nearly 12% of their cohort of 129 subjects went on to require TJR (42). These outcomes might also be related to responses to a survey by Werkman et al. of experienced TMJ surgeons regarding discectomy (43). In this study, 59 surgeons (nearly 34% response rate) did not endorse the use of discectomy as initial treatment for internal derangement, but more often for cases involving disc perforation or those cases refractory to initial minimally invasive treatment. Indeed, 85% of surgeons did not consider discectomy a useful surgery in this survey. Respondents indicated concerns for progressive DJD and potential ankylosis in terms of adverse effects of discectomy. This was consistent with our findings in the Mayo Clinic cohort. However, it should be emphasized that the survey data is based on opinions and estimates of experienced surgeons, and the studies by Ellis et al. (42), Werkman et al. (43), and the Mayo Clinic cohort do not represent prospectively-collected data on patients undergoing discectomy.
Overall, alloplastic TJR has had favorable outcomes in the management of severe anatomic mutilation and disfunction, and it has gained popularity for simulating normal anatomy, restoring vertical dimension, lacking donor site morbidity, and reducing operation time (4). In a study done by Hawkins et al. (44) to evaluate the TMJ surgeon’s preference for autogenous CCG or alloplastic prostheses, they reported on a survey of TMJ surgeons wherein 95.5% of those who completed the survey currently preferred alloplastic TMJ reconstruction over autogenous CCG reconstruction. 86.4% preferred custom-made prostheses, while 9.1% preferred stock TMJ prostheses. In listing reasons for preference for alloplastic TMJ reconstruction, 69.8% chose the ability to achieve better and moVre predictable outcomes, while 34.9% had prior experience with CCG complications including donor site morbidity, overgrowth, and postoperative facial asymmetry.
Many limitations of this study bear discussion. First, the limited number of ankylosis patients included in most studies published, and the difficulty in obtaining discrete data regarding ankylosis specifically makes it difficult to draw conclusions without aggregating all of these studies. Even with this collection of studies, data is far from uniform, with many studies not reporting on complications, etiology, prior surgeries, or even the type of alloplastic prosthesis used. Part of this is related to standards in publication or the purpose of the study, while part of it is due to the retrospective nature of the studies available. Fortunately, many studies did address these issues and furnish both the surgeon and the patient an overall picture that, in these more thorough studies, outcomes are generally very good and major complications are rare.
As noted in Table 1, some of the studies appear to contain overlapping patient populations and data, so the reader must be aware that all patients reported may not be unique in all studies. In addition, there was heterogeneity in types of prostheses utilized, although most of the studies reported on FDA-approved prostheses, a minority utilized less well-established or tested prostheses. Some studies also reported on multiple types of prostheses without separating data to be able to determine, for example, whether a Biomet prosthesis fared better or worse than a Christensen prosthesis. Furthermore, many impactful and potentially useful studies with larger sample sizes had different and mixed TMJ diagnoses and did not provide specific ankylosis data, therefore, these studies were excluded. Despite this limitation, this paper contains a relatively large sample size and a detail-oriented review of the management of ankylosis with alloplastic total joint replacement.
Many papers reported on whether or not the subjects had undergone prior TMJ surgery. However, it was unclear in many cases what type of surgery (i.e., arthroscopy, type of open surgery, or prior reconstruction) had taken place. Due to the lack of uniform data and therefore difficulty in comparison and presentation, this data was not analyzed. The discerning and interested reader to review these publications to better understand the circumstances of patient care, surgeries performed, and complications encountered.
Lastly, although MIO and complications are important outcomes to measure, many studies do not report more complete, patient-reported outcomes that illustrate the true patient experience and outcome in terms of jaw function, pain, and impact on social, occupational, and recreational activities. Those that do report quality of life, such as Wolford et al. (5), provide much-needed insights but are still limited in evaluation of a broader patient experience. This is a general weakness with much of the TMJ surgery literature, and the authors are optimistic that this will be rectified in future publications. In addition, we do not feel that missing this data means the outcomes are not favorable or that they should be ignored, simply that outcomes in future studies could be more thorough in depicting the patient experience.
Conclusions
A review of the authors’ case series as well as the available applicable published scientific literature supports treatment of TMJ ankylosis using TJR with an FDA-approved prosthesis. This treatment appears to consistently provide improved range of motion and function, with an acceptable rate of adverse events, including reankylosis. Data regarding non-FDA-approved devices for TJR are needed to support similar conclusions regarding their use.
Acknowledgments
Funding: None.
Provenance and Peer Review: This article was commissioned by the Guest Editor (Louis Mercuri) for the series “Indications for Alloplastic TMJ Replacement in Maxillofacial Surgery—an evidence-based review of the literature” published in Frontiers of Oral and Maxillofacial Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA-ScR reporting checklist. Available at https://fomm.amegroups.com/article/view/10.21037/fomm-22-15/rc
Peer Review File: Available at https://fomm.amegroups.com/article/view/10.21037/fomm-22-15/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-22-15/coif). The series “Indications for Alloplastic TMJ Replacement in Maxillofacial Surgery—an evidence-based review of the literature” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Mayo Clinic Institutional Review Board (No. 21-001585) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kazanjian VH. Ankylosis of the temporomandibular joint. American Journal of Orthodontics and Oral Surgery 1938;24:1181-206. [Crossref]
- Movahed R, Mercuri LG. Management of temporomandibular joint ankylosis. Oral Maxillofac Surg Clin North Am 2015;27:27-35. [Crossref] [PubMed]
- Mittal N, Goyal M, Sardana D, et al. Outcomes of surgical management of TMJ ankylosis: A systematic review and meta-analysis. J Craniomaxillofac Surg 2019;47:1120-33. [Crossref] [PubMed]
- Mercuri LG. The use of alloplastic prostheses for temporomandibular joint reconstruction. J Oral Maxillofac Surg 2000;58:70-5. [Crossref] [PubMed]
- Wolford L, Movahed R, Teschke M, et al. Temporomandibular Joint Ankylosis Can Be Successfully Treated With TMJ Concepts Patient-Fitted Total Joint Prosthesis and Autogenous Fat Grafts. J Oral Maxillofac Surg 2016;74:1215-27. [Crossref] [PubMed]
- Egemen O, Ozkaya O, Filinte GT, et al. Two-stage total prosthetic reconstruction of temporomandibular joint in severe and recurrent ankylosis. J Craniofac Surg 2012;23:e520-4. [Crossref] [PubMed]
- Treatment of Temporomandibular Joint Ankylosis with Total Prosthetic Joint Reconstruction [Internet]. 2022.
- Alessandra M, Braconi A, Noto F, et al. Temporo-mandibular joint reconstruction with alloplastic prostheses in the TMJ ankylosis: Our experience. Int J of Clin Dent 2012;5:393-402.
- Amarista FJ, Jones JP, Brown Z, et al. Outcomes of total joint alloplastic reconstruction in TMJ ankylosis. Oral Surg Oral Med Oral Pathol Oral Radiol 2022;134:135-42. [Crossref] [PubMed]
- Balon P, Vesnaver A, Kansky A, et al. Treatment of end stage temporomandibular joint disorder using a temporomandibular joint total prosthesis: The Slovenian experience. J Craniomaxillofac Surg 2019;47:60-5. [Crossref] [PubMed]
- Bhardwaj Y, Arya S. Post-Ankylotic Temporomandibular Joint Reconstruction Using Autogenous/Alloplastic Materials: Our Protocol and Treatment Outcomes in 22 Patients. Craniomaxillofac Trauma Reconstr 2016;9:284-93. [Crossref] [PubMed]
- Bhargava D, Neelakandan RS, Sharma Y, et al. Predictability and Feasibility of Total Alloplastic Temporomandibular Joint Reconstruction using DARSN TM Joint Prosthesis for patients in Indian subcontinent-A prospective clinical study. J Stomatol Oral Maxillofac Surg 2020;121:2-8. [Crossref] [PubMed]
- Brabyn PJ, Rodríguez Campo FJ, Escorial V, et al. New advances in customized TMJ prosthesis: Our experience at La Princesa University Hospital. Revista Espanola de Cirugia Oral y Maxilofacial 2019;41:167-71.
- Chowdhury SKR, Saxena V, Rajkumar K, et al. Evaluation of Total Alloplastic Temporomandibular Joint Replacement in TMJ Ankylosis. J Maxillofac Oral Surg 2019;18:293-8. [Crossref] [PubMed]
- Gerbino G, Zavattero E, Berrone S, et al. One stage treatment of temporomandibular joint complete bony ankylosis using total joint replacement. J Craniomaxillofac Surg 2016;44:487-92. [Crossref] [PubMed]
- Gruber EA, McCullough J, Sidebottom AJ. Medium-term outcomes and complications after total replacement of the temporomandibular joint. Prospective outcome analysis after 3 and 5 years. Br J Oral Maxillofac Surg 2015;53:412-5. [Crossref] [PubMed]
- Gundlach KK. Ankylosis of the temporomandibular joint. J Craniomaxillofac Surg 2010;38:122-30. [Crossref] [PubMed]
- Haq J, Patel N, Weimer K, et al. Single stage treatment of ankylosis of the temporomandibular joint using patient-specific total joint replacement and virtual surgical planning. Br J Oral Maxillofac Surg 2014;52:350-5. [Crossref] [PubMed]
- Hu Y, Zhang L, He D, et al. Simultaneous treatment of temporomandibular joint ankylosis with severe mandibular deficiency by standard TMJ prosthesis. Sci Rep 2017;7:45271. [Crossref] [PubMed]
- Jones RH. Temporomandibular joint reconstruction with total alloplastic joint replacement. Aust Dent J 2011;56:85-91. [Crossref] [PubMed]
- Kim JH, Park BH, Yoo MS, et al. Stability of the Natural Joint Side in Unilateral Alloplastic Total Temporomandibular Joint Replacement Using a Ready-Made System. Applied Sci 2021;11:3935. [Crossref]
- Kunjur J, Niziol R, Matthews NS. Quality of life: patient-reported outcomes after total replacement of the temporomandibular joint. Br J Oral Maxillofac Surg 2016;54:762-6. [Crossref] [PubMed]
- Linsen SS, Reich RH, Teschke M. Maximum voluntary bite force in patients with alloplastic total TMJ replacement--a prospective study. J Craniomaxillofac Surg 2013;41:423-8. [Crossref] [PubMed]
- Loveless TP, Bjornland T, Dodson TB, et al. Efficacy of temporomandibular joint ankylosis surgical treatment. J Oral Maxillofac Surg 2010;68:1276-82. [Crossref] [PubMed]
- Machon V, Hirjak D, Beno M, et al. Total alloplastic temporomandibular joint replacement: the Czech-Slovak initial experience. Int J Oral Maxillofac Surg 2012;41:514-7. [Crossref] [PubMed]
- Mani B, Balasubramaniam S, Balasubramanian S, et al. Role of Custom-Made Prosthesis for Temporomandibular Joint Replacement in Unilateral Ankylosis - An Evaluative Study. Ann Maxillofac Surg 2020;10:344-52. [Crossref] [PubMed]
- Mercuri LG, Ali FA, Woolson R. Outcomes of total alloplastic replacement with periarticular autogenous fat grafting for management of reankylosis of the temporomandibular joint. J Oral Maxillofac Surg 2008;66:1794-803. [Crossref] [PubMed]
- Pearce CS, Cooper C, Speculand B. One stage management of ankylosis of the temporomandibular joint with a custom-made total joint replacement system. Br J Oral Maxillofac Surg 2009;47:530-4. [Crossref] [PubMed]
- Rikhotso RE, Sekhoto MG. Alloplastic Total Temporomandibular Joint Reconstruction: A 10-Year Experience of the University of the Witwatersrand, Johannesburg. J Craniofac Surg 2021;32:1658-63. [PubMed]
- Roy Chowdhury SK, Saxena V, Rajkumar K, et al. Clinical outcome of total alloplastic temporomandibular joint reconstruction in cases of recurrent ankylosis with emphasis on pitfalls: A retrospective analysis. J Oral Maxillofac Surg Med Pathol 2019;31:20-4. [Crossref]
- Roychoudhury A, Yadav P, Alagarsamy R, et al. Outcome of Stock Total Joint Replacement With Fat Grafting in Adult Temporomandibular Joint Ankylosis Patients. J Oral Maxillofac Surg 2021;79:75-87. [Crossref] [PubMed]
- Sahdev R, Wu BW, Anderson N, et al. A Retrospective Study of Patient Outcomes After Temporomandibular Joint Replacement With Alloplastic Total Joint Prosthesis at Massachusetts General Hospital. J Oral Maxillofac Surg 2019;77:280-8. [Crossref] [PubMed]
- Westermark A. Total reconstruction of the temporomandibular joint. Up to 8 years of follow-up of patients treated with Biomet(®) total joint prostheses. Int J Oral Maxillofac Surg 2010;39:951-5. [Crossref] [PubMed]
- Yadav P, Roychoudhury A, Bhutia O. Strategies to reduce re-ankylosis in temporomandibular joint ankylosis patients. Br J Oral Maxillofac Surg 2021;59:820-5. [Crossref] [PubMed]
- Zou L, Zhang L, He D, et al. Clinical and Radiologic Follow-Up of Zimmer Biomet Stock Total Temporomandibular Joint Replacement After Surgical Modifications. J Oral Maxillofac Surg 2018;76:2518-24. [Crossref] [PubMed]
- Al-Moraissi EA, El-Sharkawy TM, Mounair RM, et al. A systematic review and meta-analysis of the clinical outcomes for various surgical modalities in the management of temporomandibular joint ankylosis. Int J Oral Maxillofac Surg 2015;44:470-82. [Crossref] [PubMed]
- Saeed N, Hensher R, McLeod N, et al. Reconstruction of the temporomandibular joint autogenous compared with alloplastic. Br J Oral Maxillofac Surg 2002;40:296-9. [Crossref] [PubMed]
- Györi E, Przestrzelski C, Pona I, et al. Quality of life and functional assessment of facial palsy patients: A questionnaire study. Int J Surg 2018;55:92-7. [Crossref] [PubMed]
- Saeed NR, McLeod NMH. Predictive risk factors for facial nerve injury in temporomandibular joint replacement surgery. Br J Oral Maxillofac Surg 2021;59:1243-7. [Crossref] [PubMed]
- Wolford LM, Karras SC. Autologous fat transplantation around temporomandibular joint total joint prostheses: preliminary treatment outcomes. J Oral Maxillofac Surg 1997;55:245-51; discussion 251-2. [Crossref] [PubMed]
- Tang W, Long J, Feng F, et al. Condyle replacement after tumor resection: comparison of individual prefabricated titanium implants and costochondral grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:147-52. [Crossref] [PubMed]
- Ellis OG, Tocaciu S, McKenzie DP, et al. Risk Factors Associated With Poor Outcomes Following Temporomandibular Joint Discectomy and Fat Graft. J Oral Maxillofac Surg 2021;79:2448-54. [Crossref] [PubMed]
- Werkman DF, Mercuri LG, Troost JP, et al. An International Survey on Temporomandibular Joint Surgeon’s Implementation and Management of Discectomy in Treating Temporomandibular Joint Internal Derangement. J Oral Maxillofac Surg 2021;79:1423-33. [Crossref] [PubMed]
- Hawkins A, Mercuri LG, Miloro M. Are Rib Grafts Still Used for Temporomandibular Joint Reconstruction? J Oral Maxillofac Surg 2020;78:195-202. [Crossref] [PubMed]
doi: 10.21037/fomm-22-15
Cite this article as: Ahmed A, Hassett LC, Fillmore WJ. Treatment of temporomandibular joint ankylosis with total prosthetic joint reconstruction: a case series and scoping review of the literature. Front Oral Maxillofac Med 2023;5:36.