
Ảnh người yêu che mặt đẹp
Bạn đang tìm kiếm những bức ảnh người yêu che mặt đẹp và ấn tượng để chia sẻ trên mạng xã hội? Dưới đây là bộ sưu tập những bức ảnh che mặt đẹp nhất hiện nay để bạn tham khảo.
Nhiều người hiện nay muốn khoe người yêu nhưng lại không muốn lộ diện của nửa kia. Họ thường chọn cách chia sẻ ảnh bìa, story hay hình đại diện với người yêu che mặt. Nếu bạn đang tìm kiếm những bức ảnh người yêu che mặt đẹp và độc đáo, hãy tham khảo bộ sưu tập hình ảnh mà Mytour giới thiệu dưới đây.

Dưới đây là bộ ảnh người yêu che mặt đẹp nhất mà Mytour đã tổng hợp, mời bạn cùng khám phá và sử dụng để làm mới ảnh đại diện hoặc story của mình.

Những bức ảnh chụp cùng người yêu với cách che mặt đẹp nhất

Những bức ảnh chụp cùng người yêu với mặt được che đẹp mắt

Hình ảnh chụp cùng người yêu, mặt che khéo léo

Ảnh người yêu che mặt cực kỳ ấn tượng

Ảnh chụp chung với người yêu, mặt được che kín đáo

Những bức ảnh che mặt của người yêu đẹp nhất

Hình ảnh người yêu che mặt khi ôm nhau đẹp

Ảnh người yêu che mặt tuyệt đẹp trên bãi cát biển

Ảnh người yêu che mặt đẹp tại bãi biển

Ảnh người yêu che mặt tuyệt đẹp trên phố

Hình ảnh người yêu che mặt vừa đẹp vừa dễ thương

Ảnh người yêu che mặt đẹp và đáng yêu

Những bức ảnh người yêu che mặt đẹp mắt

Ảnh người yêu che mặt khi đi dạo thật đẹp

Hình ảnh người yêu che mặt khi đi chơi

Ảnh người yêu che mặt tuyệt đẹp trong chuyến du lịch

Hình ảnh người yêu che mặt khi du lịch
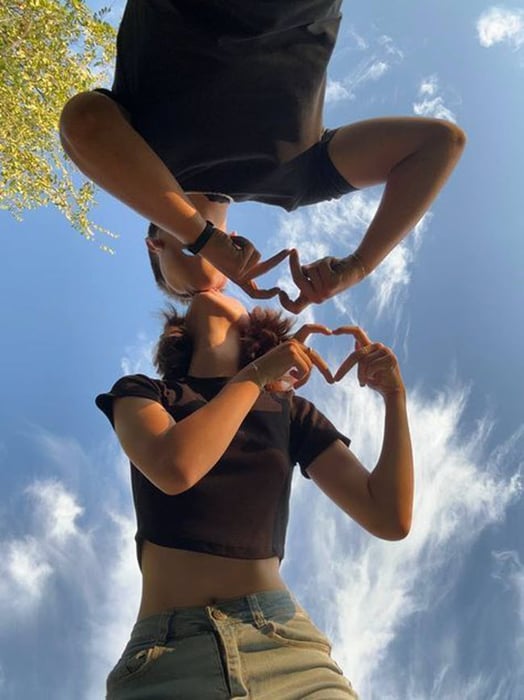
Ảnh người yêu che mặt dưới bầu trời rộng lớn

Hình ảnh người yêu che mặt dưới ánh hoàng hôn lãng mạn

Ảnh người yêu che mặt khi tận hưởng buổi đi chơi

Ảnh người yêu che mặt khi khóa môi đẹp nhất

Ảnh người yêu che mặt khi khóa môi trước gương

Hình ảnh người yêu che mặt từ phía sau lưng

Ảnh người yêu che mặt đẹp và cuốn hút

Ảnh người yêu che mặt với nền bầu trời tuyệt đẹp

Hình ảnh người yêu giấu mặt đẹp nhất

Ảnh người yêu giấu mặt thật đẹp

Ảnh người yêu giấu mặt khi ôm nhau xinh đẹp

Ảnh người yêu che mặt ấn tượng

Những bức ảnh người yêu che mặt đẹp nhất

Ảnh người yêu che mặt tuyệt đẹp

Chụp ảnh người yêu với khuôn mặt bị che

Hình ảnh người yêu che mặt khi ở bên nhau

Những bức ảnh người yêu che mặt ấn tượng nhất

Ảnh người yêu che mặt đẹp khi ôm nhau

Hình ảnh người yêu che mặt nổi bật trên phố

Ảnh người yêu che mặt tuyệt vời

Ảnh người yêu che mặt khi đi ăn

Hình ảnh người yêu che mặt khi dạo chơi

Những bức ảnh người yêu che mặt khi vui chơi

Ảnh người yêu che mặt với khoảnh khắc khóa môi đẹp nhất

Ảnh người yêu che mặt với khoảnh khắc khóa môi tuyệt đẹp

Những hình ảnh người yêu che mặt

Ảnh người yêu giấu mặt đẹp nhất

Hình ảnh người yêu giấu mặt tuyệt đẹp

Ảnh người yêu giấu mặt

Ảnh người yêu che mặt cực kỳ đẹp

Ảnh người yêu che mặt đẹp nhất

Ảnh người yêu che mặt đẹp

Ảnh người yêu che mặt khi đi dạo đẹp

Ảnh người yêu che mặt trong chuyến du lịch tuyệt vời

Ảnh người yêu che mặt khi vui chơi

Ảnh người yêu che mặt trong thang máy

Ảnh người yêu che mặt
Bạn vừa xem các hình ảnh người yêu che mặt đẹp, từ những bức ảnh chụp cùng nhau khi đi chơi, du lịch, đến những khoảnh khắc trên đường. Hy vọng bạn sẽ yêu thích những hình ảnh này mà Mytour đã chia sẻ. Cảm ơn bạn đã theo dõi bài viết.
Nội dung được phát triển bởi đội ngũ Mytour với mục đích chăm sóc khách hàng và chỉ dành cho khích lệ tinh thần trải nghiệm du lịch, chúng tôi không chịu trách nhiệm và không đưa ra lời khuyên cho mục đích khác.
Nếu bạn thấy bài viết này không phù hợp hoặc sai sót xin vui lòng liên hệ với chúng tôi qua email [email protected]
Admin
Link nội dung: https://pi-web.eu/anh-nguoi-yeu-che-mat-dep-1736130009-a3724.html