Nụ cười là một trong những yếu tố quan trọng trong giao tiếp và tạo ấn tượng với người khác. Một nụ cười đẹp không chỉ tăng tính thân thiện mà còn nâng cao sự tự tin. Bài viết này sẽ tập trung vào các yếu tố của một nụ cười đẹp và danh sách những người nổi tiếng có nụ cười cuốn hút nhất trên thế giới. Ngoài ra, hãy cùng nha khoa Volcano khám phá hình ảnh những nụ cười đẹp tại Việt Nam và cách cười đẹp dành cho cả nam và nữ.

Các yếu tố của 1 nụ cười đẹp
Để có một nụ cười đẹp, có một số yếu tố quan trọng cần được chú trọng:
Răng trắng đều:
- Răng trắng và đều là yếu tố quan trọng. Cười với một bộ răng đẹp sẽ tạo nên sự ấn tượng tích cực và thu hút sự chú ý.
Đôi môi đẹp:
- Đôi môi đẹp và phù hợp với khuôn mặt giúp nụ cười trở nên cuốn hút hơn.
Khuôn mặt đối xứng:
- Khuôn mặt đối xứng làm cho nụ cười trở nên hài hòa và hấp dẫn hơn.
Biểu cảm tổng thể và ngôn ngữ cơ thể:
- Nụ cười cần phải đi kèm với sự tự tin và tính thân thiện trong cử chỉ và ngôn ngữ cơ thể.
Neymar Jr:
- Là cầu thủ bóng đá nổi tiếng người Brazil, có nụ cười rạng rỡ và hấp dẫn.

Tom Cruise:
- Diễn viên Hollywood với nụ cười quyến rũ và tươi tắn.

Leonardo DiCaprio:
- Ngôi sao điện ảnh hạng A có nụ cười năng động và tỏa nắng.

Park Bo Gum:
- Diễn viên Hàn Quốc với nụ cười tươi tắn và hồn nhiên.

Kim Bum:
- Nam diễn viên nổi tiếng xứ Hàn với nụ cười hút hồn.

Song Joong Ki:
- Một diễn viên xuất sắc khác của Hàn Quốc với nụ cười ấm áp.

Park Seo Joon:
- Nam diễn viên quyến rũ và lôi cuốn.

Những người phụ nữ có nụ cười đẹp nhất thế giới
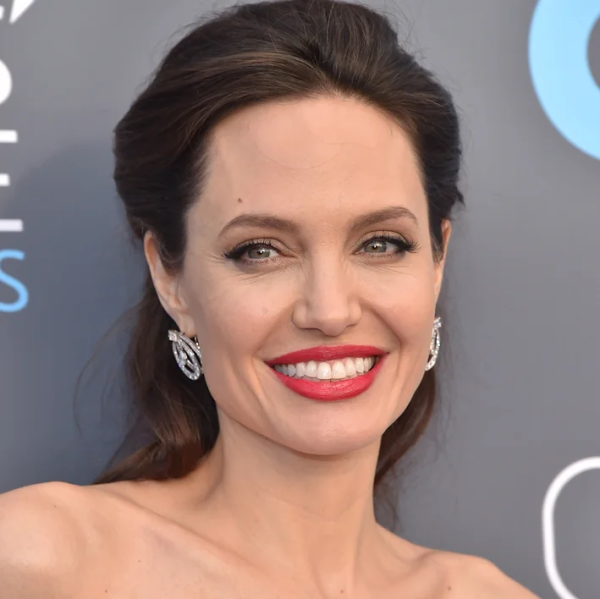
Angelina Jolie:
- Nữ diễn viên kiêm nhà hoạt động nhân đạo với nụ cười quyến rũ và thu hút.
Julianna Margulies:
- Nữ diễn viên nổi tiếng với nụ cười tươi tắn và duyên dáng.

Cameron Diaz:
- Ngôi sao Hollywood với nụ cười rạng rỡ và nhiệt tình.

Jennifer Lopez:
- Ca sĩ, diễn viên và nhà thiết kế người Mỹ với nụ cười tỏa nắng.

Jennifer Garner:
- Nữ diễn viên đa năng với nụ cười tươi tắn và thân thiện.

Nancy (Momoland):
- Thành viên nhóm nhạc nữ Hàn Quốc với nụ cười ngọt ngào.

Siêu mẫu Christie Brinkley:
- Người mẫu và diễn viên nổi tiếng với nụ cười quyến rũ.
Miranda Kerr:
- Siêu mẫu nổi tiếng với nụ cười thần thái.

Emma Watson:
- Nữ diễn viên xinh đẹp và thông minh với nụ cười tỏa sáng.

Son Ye Jin:
- Nữ diễn viên Hàn Quốc với nụ cười ấm áp và thu hút.

Gong Hyo Jin:
- Nữ diễn viên tài năng với nụ cười duyên dáng.

Hình ảnh nụ cười đẹp nhất tại Việt Nam
Hoa Hậu Tiểu Vy:
- Hoa hậu Việt Nam với nụ cười tươi tắn và quyến rũ.

Hoa hậu Đỗ Thị Hà:
- Người đẹp đăng quang cuộc thi Hoa hậu Việt Nam với nụ cười duyên dáng.

Khả Ngân:
- Nữ diễn viên trẻ có nụ cười ngọt ngào và thân thiện.

Midu:
- Nữ diễn viên và người mẫu với nụ cười tỏa nắng.

Nhã Phương:
- Nữ diễn viên nổi tiếng với nụ cười ấm áp và hồn nhiên.

Phạm Thùy Trang (Xoài non):
- Cựu thành viên nhóm nhạc Mắt Ngọc với nụ cười dễ thương.

Ngọc Nữ Tăng Thanh Hà:
- Nữ diễn viên nổi tiếng và duyên dáng.

Ninh Dương Lan Ngọc:
- Nữ diễn viên tài năng với nụ cười tỏa sáng.

Noo Phước Thịnh:
- Ca sĩ nam đẹp trai và có nụ cười cuốn hút.

Hướng dẫn cách cười đẹp cho nam và nữ cực đơn giản
Có một số bí quyết đơn giản để có một nụ cười đẹp và cuốn hút. Dưới đây là những gợi ý hữu ích để bạn tự tin tỏa sáng với nụ cười của mình.
Chăm sóc răng và vệ sinh miệng đều đặn:
- Việc chải răng ít nhất hai lần mỗi ngày và sử dụng chỉ dạy giúp loại bỏ mảng bám và vi khuẩn, giữ cho răng miệng luôn sạch sẽ và khỏe mạnh.
>>Tham khảo: Cách vệ sinh răng niềng an toàn hiệu quả
Tập luyện cười và rèn cho cơ môi và khuôn mặt:
- Thực hiện các bài tập cười như cười to, cười nhỏ, hay mỉm cười giúp rèn cho cơ môi và khuôn mặt, giúp nụ cười tự nhiên và rạng rỡ hơn.
Chăm sóc đôi môi:
- Dưỡng ẩm đôi môi thường xuyên để tránh tình trạng khô, nứt nẻ, và chọn son môi phù hợp với màu răng và khuôn mặt.
Hạn chế sử dụng thuốc lá và thức uống có màu đậm:
- Thuốc lá và thức uống như cà phê, trà, và rượu có thể làm bẩn răng và làm mất đi sự trắng sáng tự nhiên của răng.
Hãy tự tin và mỉm cười thường xuyên:
- Tự tin là điều quan trọng, mỉm cười thường xuyên giúp tạo nên sự gần gũi và thân thiện với mọi người xung quanh.
Tìm hiểu về kỹ thuật cười trong hình ảnh:
- Tìm hiểu cách tạo cảm giác tự nhiên và thu hút trong các bức ảnh khi cười để có những bức hình nụ cười đẹp và tự tin.
Tạo không gian thoải mái khi cười:
- Hãy chọn môi trường thoải mái và vui vẻ để cười, điều này giúp nụ cười của bạn tỏa sáng tự nhiên và cuốn hút hơn.
Tự yêu thương nụ cười của mình:
- Hãy trân trọng và yêu thương nụ cười của bạn, bởi nụ cười đẹp là điều làm bạn trở nên đặc biệt và đáng yêu.
Bằng cách áp dụng những bí quyết trên, bạn sẽ tự tin và thu hút với nụ cười đẹp tự nhiên của mình. Hãy để nụ cười trở thành “vũ khí” tuyệt vời của bạn trong cuộc sống hàng ngày.
Bí quyết để có một nụ cười đẹp
Có một số bí quyết quan trọng giúp duy trì và tạo nên một nụ cười đẹp và rạng rỡ. Dưới đây là những điều bạn nên lưu ý để có một nụ cười tự tin và cuốn hút.
Đi khám chữa răng định kỳ:
- Điều này là một trong những yếu tố quan trọng nhất để có một nụ cười khỏe mạnh và đẹp. Hãy đi khám chữa răng định kỳ ít nhất hai lần một năm để kiểm tra và làm sạch răng miệng. Nha sĩ sẽ giúp bạn phát hiện và điều trị các vấn đề răng miệng kịp thời, giúp bảo vệ răng và nụ cười của bạn.
Làm trắng răng:
- Nụ cười sáng bóng và trắng sẽ tạo ấn tượng tích cực. Bạn có thể sử dụng các sản phẩm làm trắng răng chứa peroxide hoặc đến nha sĩ để làm trắng răng chuyên nghiệp. Hãy chọn phương pháp phù hợp với bạn và tuân thủ hướng dẫn sử dụng để đạt hiệu quả tốt nhất.
>>Tham khảo:
- Cách làm trắng răng tại nhà đơn giản và hiệu quả nhất cho nụ cười tươi tắn
- Cách làm trắng răng bằng chanh tại nhà hiệu quả
- Cách làm trắng răng bằng baking soda đơn giản và hiệu quả
Chọn màu son phù hợp:
- Màu son môi có thể làm nổi bật nụ cười và tạo cảm giác tự tin. Hãy chọn màu son phù hợp với màu răng và khuôn mặt của bạn để tôn lên vẻ đẹp tự nhiên của nụ cười.
Giữ vệ sinh miệng sau mỗi bữa ăn:
- Sau mỗi bữa ăn, hãy rửa miệng bằng nước hoặc súc miệng bằng nước muối pha loãng để loại bỏ thức ăn và vi khuẩn trong miệng. Điều này giúp giữ cho hơi thở thơm mát và ngăn ngừa các vấn đề về răng miệng.
Tự tin mỉm cười:
- Tự tin là điều quan trọng nhất khi mỉm cười. Hãy tự yêu thương và tự tin với nụ cười của mình, và mỉm cười thường xuyên trong cuộc sống hàng ngày. Nụ cười tự tin sẽ làm bạn trở nên cuốn hút và gần gũi với mọi người xung quanh.
Hãy cười một cách tự nhiên và thường xuyên:
- Cười giúp giải tỏa căng thẳng và mang lại cảm giác hạnh phúc. Hãy cười thường xuyên và tự nhiên, điều này giúp nụ cười của bạn tỏa sáng và cuốn hút hơn.
Học cách tạo cảm giác tự nhiên trong ảnh khi cười:
- Khi chụp ảnh, hãy tập trung vào cách tạo cảm giác tự nhiên và duyên dáng khi cười. Điều này giúp bạn có những bức ảnh nụ cười đẹp và tự tin.
Nhớ rằng, nụ cười đẹp không chỉ tạo nên vẻ đẹp bên ngoài mà còn phản ánh sự tự tin và tính cách của bạn. Hãy yêu thương và chăm sóc nụ cười của mình, và sẵn lòng tỏa sáng với nụ cười đẹp tự nhiên và tự tin của bạn.
Kết luận
Nụ cười đẹp không chỉ làm cho bạn trở nên quyến rũ và tự tin hơn mà còn tạo ra một ấn tượng tốt với mọi người xung quanh. Hãy chăm sóc và yêu thương nụ cười của bạn, và đừng ngại tỏ ra vui vẻ và tự tin bằng nụ cười của mình. Nụ cười là biểu tượng của sự hạnh phúc và tình yêu đời, hãy để nó luôn tỏa sáng trong cuộc sống của bạn.
>>Tham khảo:
- Niềng răng mắc cài pha lê có tốt không? Loại nào tốt? Giá bao nhiêu?
- Niềng răng khểnh có nên không? Thời gian niềng răng khấp khểnh bao lâu?
- Quy trình niềng răng diễn ra như thế nào? Các bước chuẩn nhất

