
10 bức hình về không gian ấn tượng nhất năm 2024
(Dân trí) - Từ bão mặt trời đến cảnh mưa sao băng diễn ra tại kỳ quan Stonehenge, đây là 10 bức ảnh không gian ấn tượng nhất, do tạp chí Livescience bình chọn.
"Con tàu lật" và sứ mệnh lịch sử

Hình ảnh được chụp bởi rover tự hành Sora-Q cho thấy tàu đổ bộ SLIM của Nhật Bản đã hạ cánh an toàn nhưng bị lật ngược (Ảnh: JAXA).
Ngày 19/1, Cơ quan Thám hiểm Hàng không Vũ trụ Nhật Bản (JAXA) đã làm nên lịch sử khi tàu đổ bộ SLIM hạ cánh thành công lên bề mặt Mặt Trăng. Đây là lần đầu tiên Nhật Bản hạ cánh thành công và cũng là lần hạ cánh chính xác nhất trong lịch sử, chỉ cách mục tiêu khoảng 10 mét.
Tuy nhiên, SLIM hạ cánh ở tư thế lộn ngược. Bất chấp vấn đề này, tàu đã sống sót qua ba đêm lạnh giá trên Mặt Trăng trước khi mất liên lạc vào tháng 4. Rover nhỏ đi kèm, Sora-Q, đã chụp lại hình ảnh ấn tượng này.
Đôi mắt đỏ máu

Hai thiên hà xoắn ốc IC 2163 và NGC 2207 xuất hiện với hình dạng giống đôi mắt đỏ rực (Ảnh: NASA, ESA).
Hình ảnh từ kính viễn vọng Hubble và James Webb cho thấy hai thiên hà đang giao thoa. Nhờ khả năng phát hiện bước sóng hồng ngoại của Webb, hình ảnh được phối màu đỏ rực, mang vẻ ma mị. Hai thiên hà này sẽ hợp nhất thành một cấu trúc khổng lồ trong tương lai.
Bức hình selfie với Trái Đất

Tàu đổ bộ Odysseus của Intuitive Machines gửi về một bức ảnh "tự sướng" với Trái Đất vào ngày 16/2 (Ảnh: Intuitive Machines).
Ngày 15/2, công ty Intuitive Machines đã phóng tàu đổ bộ Odysseus lên Mặt Trăng. Ngày 22/2, tàu hạ cánh thành công gần cực nam Mặt Trăng, trở thành tàu tư nhân đầu tiên đạt được kỳ tích này. Trên hành trình, Odysseus đã chụp ảnh kỷ niệm từ quỹ đạo với Trái Đất ở nền.
Hồ dung nham ngoài hành tinh

Hồ dung nham trên Mặt Trăng Io của Sao Mộc được ghi lại bởi tàu Juno (Ảnh: NASA).
Io, mặt trăng của Sao Mộc, là thiên thể có hoạt động núi lửa mạnh nhất trong Hệ Mặt Trời. Tàu Juno của NASA đã chụp cận cảnh hồ dung nham Loki Patera, dài 200 km, với lớp dung nham nguội lạnh ở trung tâm và bao quanh là magma nóng chảy.
"Bàn tay của Chúa"

Hình ảnh "Bàn tay của Chúa" - một đám mây tạo sao khổng lồ - được chụp tại chòm sao Puppis (Ảnh: NASA).
NASA công bố hình ảnh về một đám mây khí khổng lồ mang hình dạng giống bàn tay. Nó được cho là hình thành bởi bức xạ từ các ngôi sao nóng trẻ hoặc vụ nổ siêu tân tinh, cấu trúc này nằm cách Trái Đất 1.300 năm ánh sáng.
Mưa sao băng ở bãi đá Stonehenge

Mưa sao băng Perseid rơi xuống bầu trời phía trên kỳ quan Stonehenge (Ảnh: Josh Dury).
Nhiếp ảnh gia Josh Dury đã chụp lại cảnh tượng kỳ diệu này bằng cách ghép 43 bức ảnh để tạo ra hình ảnh cuối cùng. Trong bức hình, kỳ quan Stonehenge, một công trình thiên văn cổ xưa, trở thành bối cảnh tuyệt đẹp trong lúc diễn ra trận mưa sao băng.
"Quái thú" ngoài thiên hà
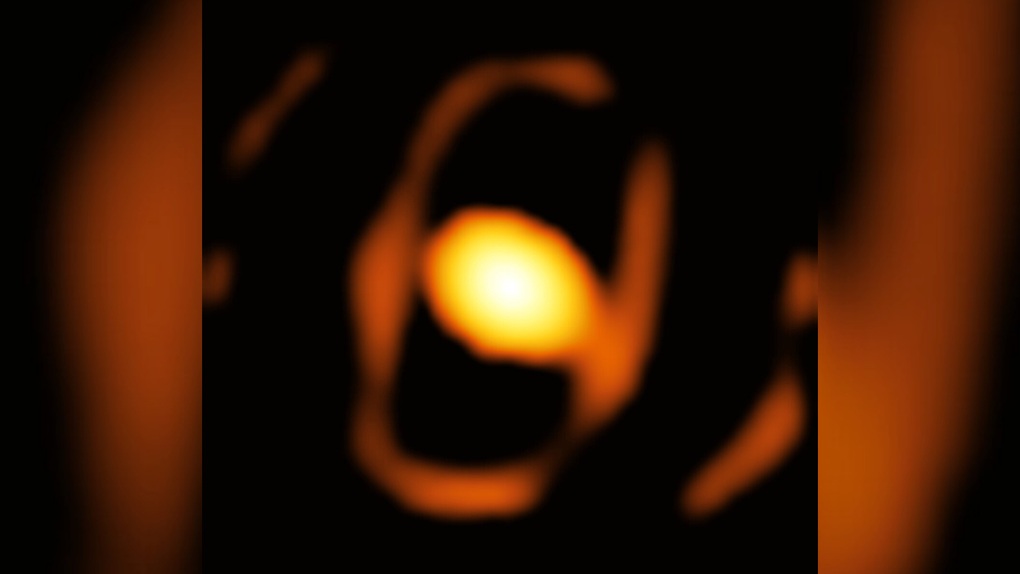
Hình ảnh đầu tiên của một ngôi sao ngoài thiên hà, WOH G64 (Ảnh: VLTI).
Kính viễn vọng VLTI tại Chile chụp được hình ảnh đầu tiên của WOH G64, một ngôi sao ngoài thiên hà. Nó có kích thước lớn hơn Mặt Trời 1.500 lần và có thể đang trên đà phát nổ trong vụ siêu tân tinh.
Mặt Trời đen và ngọn lửa hồng
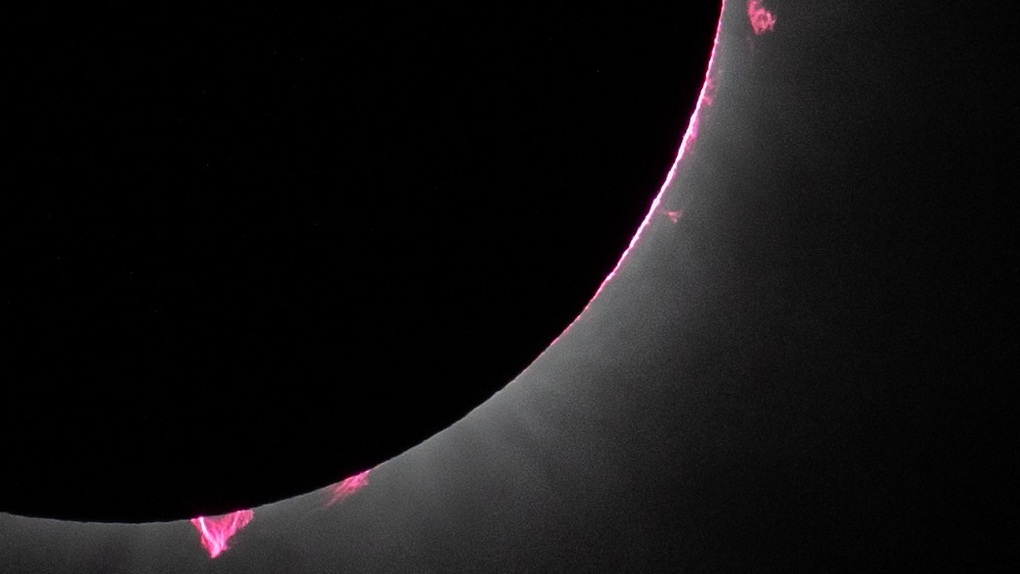
Hình ảnh các đám lửa plasma hồng bùng ra từ Mặt Trời trong nhật thực toàn phần (Ảnh: NASA).
Ngày 8/4, nhật thực toàn phần xảy ra, trải dài khắp Bắc Mỹ. Những đám plasma hồng, gọi là vệt lửa mặt trời, xuất hiện khi quan sát trên bầu trời. Hiện tượng này xảy ra do khí hydro phát sáng ở nhiệt độ cao.
Rác không gian

Ảnh chụp gần đầu tiên về một mảnh rác không gian (Ảnh: Astroscale).
Công ty Astroscale đã chụp ảnh tầng trên của tên lửa Nhật Bản H-IIA, đang quay quanh Trái Đất từ năm 2009. Đây là một vật thể có thể gây nguy hiểm cho các phi hành gia và tàu vũ trụ, và làm tắc nghẽn tầng khí quyển trên của chúng ta. Astroscale sẽ thực hiện một nhiệm vụ trong tương lai, nhằm cố gắng loại bỏ mảnh rác vũ trụ khỏi quỹ đạo bằng cách sử dụng một cánh tay robot.
Con người và vũ trụ

Tỷ phú Jared Isaacman thực hiện chuyến đi bộ không gian thương mại đầu tiên trong lịch sử (Ảnh: SpaceX).
Vào tháng 9, trong dự án Polaris Dawn, tỷ phú kiêm phi hành gia tư nhân Jared Isaacman đã bước ra khoảng không vũ trụ từ tàu SpaceX Crew Dragon để thử nghiệm bộ đồ không gian trong khoảng 10 phút. Chuyến "đi bộ ngoài không gian" lịch sử đã tạo nên một trong những bức ảnh biểu tượng của năm 2024.
Admin
Link nội dung: https://pi-web.eu/10-buc-hinh-ve-khong-gian-an-tuong-nhat-nam-2024-1735789508-a2582.html