Các mẫu nokia đời cũ như 1280, nokia 110i, 1202, nokia 1200 vẫn là một cái gì đó rất hoài niệm. Vẫn khiến chúng ta nhớ về một phần của tuổi thơ khi được dùng một trong những chiếc điện thoại đầu tiên.
Cho đến hiện tại, chúng vẫn rất được ưa chuộng, nhưng là được ưa chuộng trên màn hình điện thoại iPhone. Hình nền các mẫu điện thoại nokia màn hình trắng đen, màn hình xanh vẫn được ưa chuộng dù không đẹp mấy nhưng nó khiến người dùng như đang cầm trên tay một chiếc nokia đời cũ.
Dưới đây là danh sách hình nền nokia 1280, nokia 110i, 1202, nokia 1200, bao gồm những hình nền nokia màn hình đen trắng và hình nền nokia anime.
Tải hình nền điện thoại 1280, hình nền nokia anime, ảnh nokia anime, hình nền nokia anime iPhone
Với bộ hình nền ở dưới đây, bạn có thể tải và lưu trực tiếp về máy để lưu lại hình nền nokia 1280. Còn với bộ hình nền chỉ có màn hình trắng đen và các ký tự ở dưới sẽ có link tải với độ phân giải gốc.




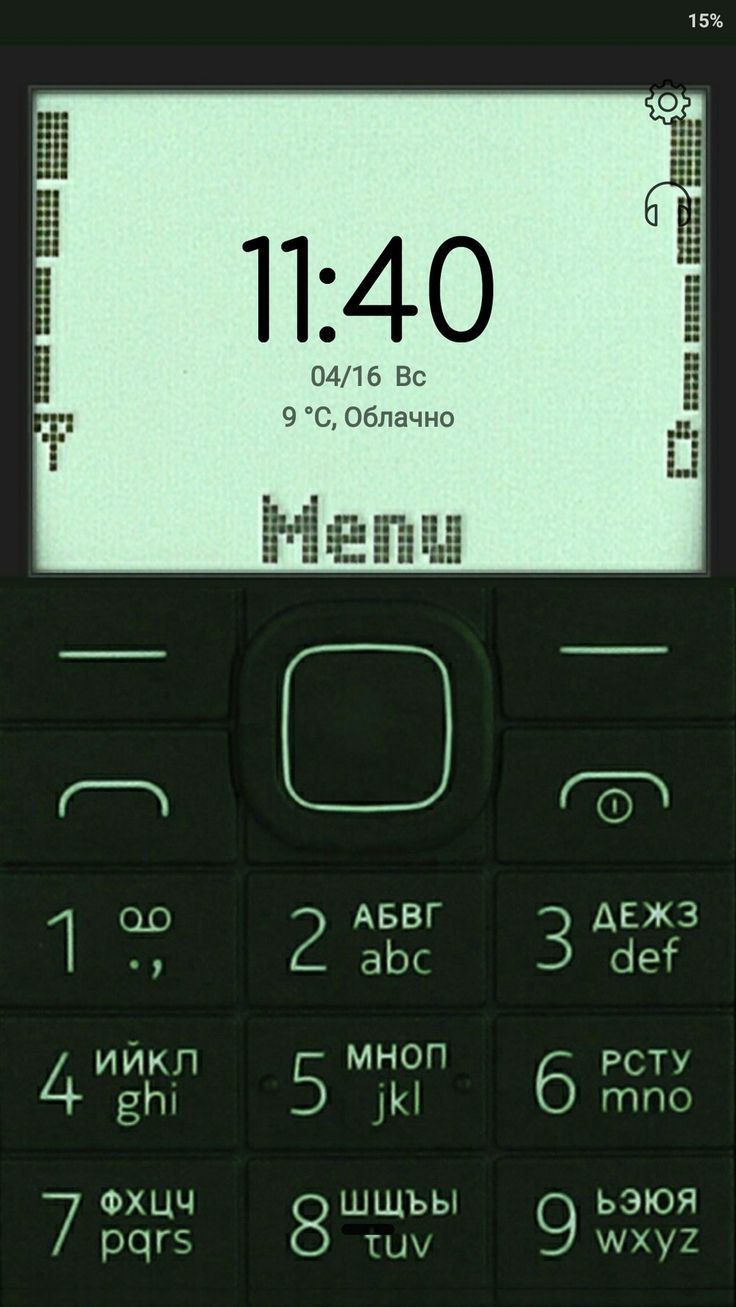


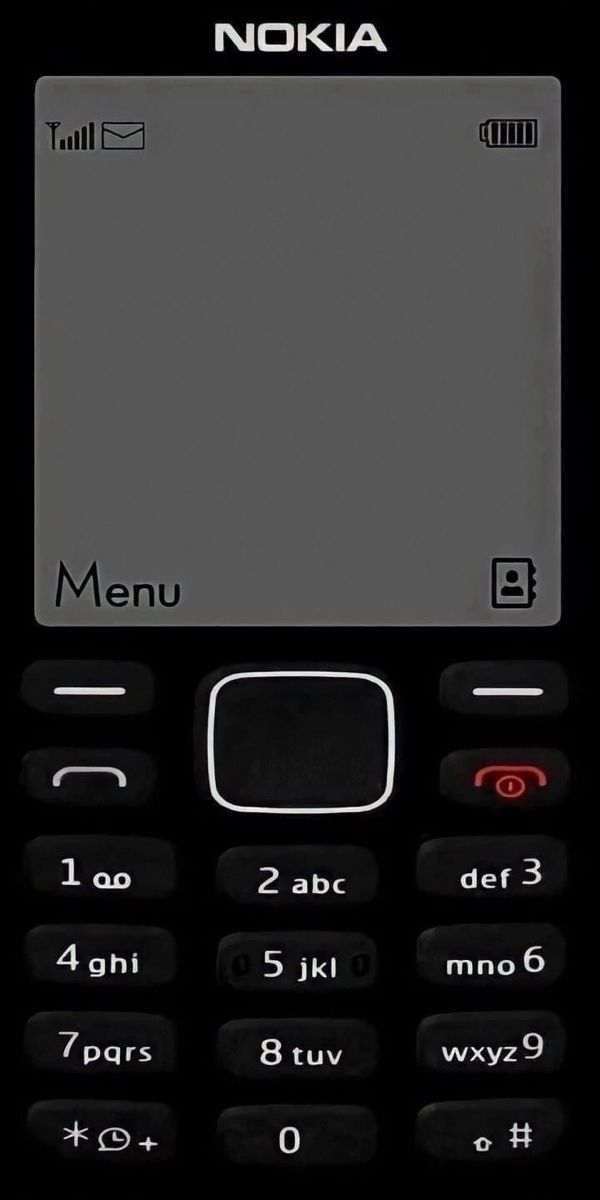
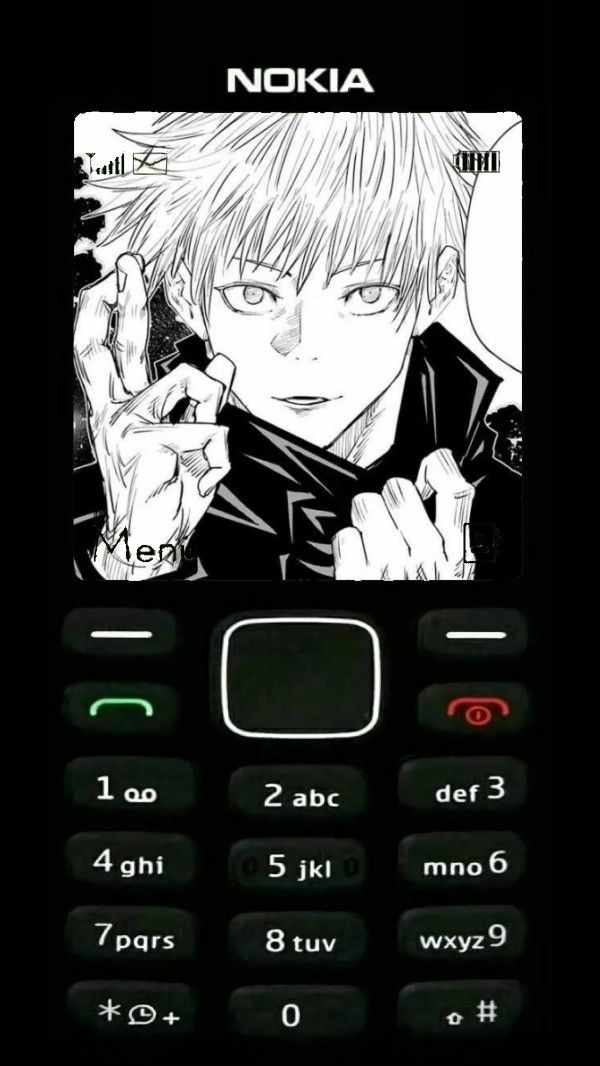
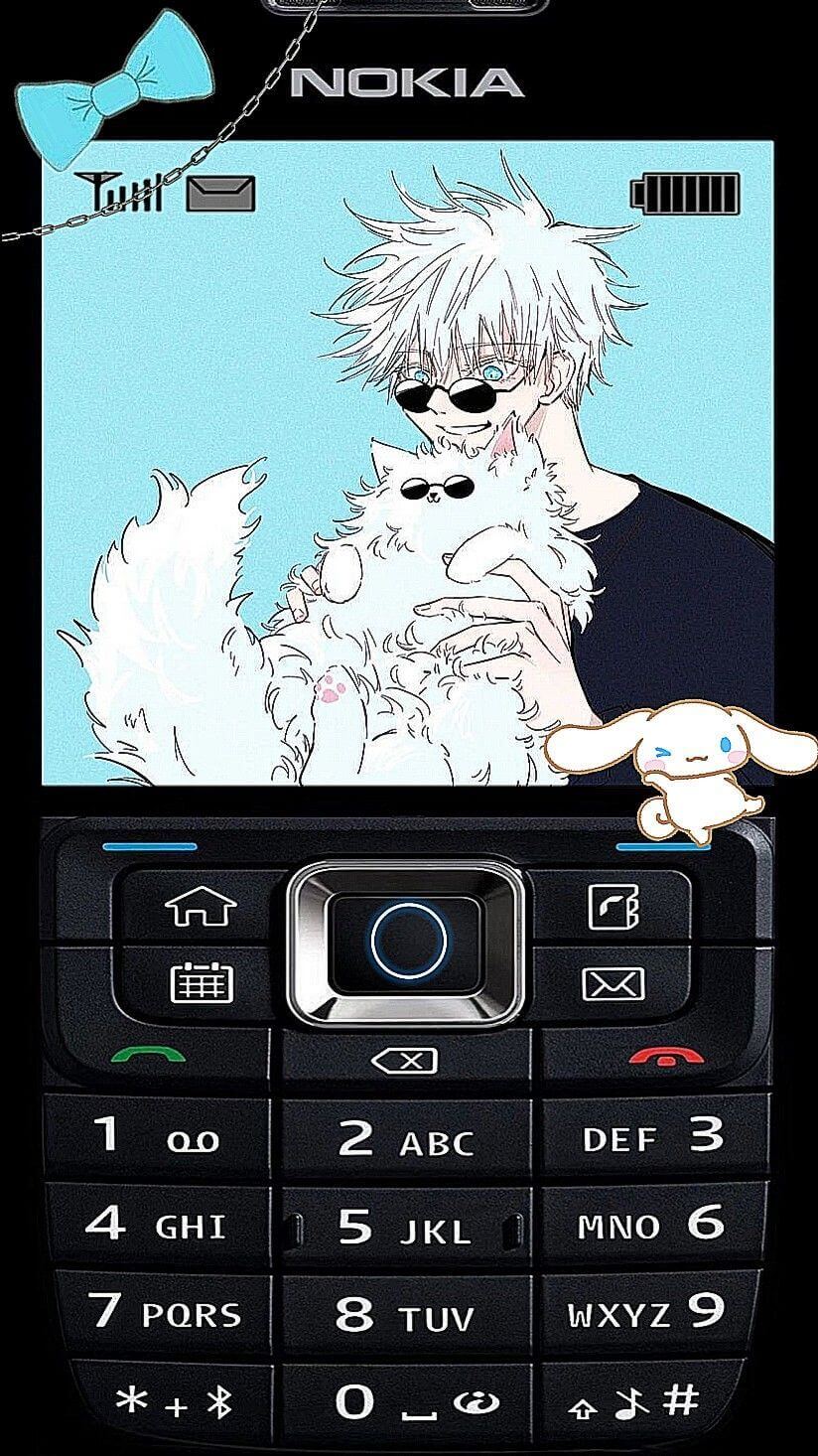











Link tải bộ hình nền nokia IP trắng đen với các ký tự
Để tải về bộ hình nền 1280 với độ phân giải mặc định, bạn hãy bấm vào link ở dưới đây. Còn list hình nền này chỉ là bộ hình nền đã được nén lại để các bạn xem thử.
Link tải hình nền 1280






Một vài điều thú vị về hãng Nokia
1) Nokia 1110 là thiết bị di động có doanh số cao nhất trong lịch sử, bán được khoảng 250 triệu chiếc.
2) Nokia đôi khi được gọi là ' aikon ' (Nokia đọc ngược) bởi những người dùng điện thoại di động không của Nokia và bởi các nhà phát triển phần mềm di động, bởi vì ' aikon ' được sử dụng trong các gói phần mềm SDK khác nhau, bao gồm SDK Symbian S60 của chính Nokia.
3) Tên Nokia bắt nguồn từ một con sông chảy qua thị trấn và nó có tên là Nokianvirta. Bản thân con sông được đặt tên theo từ tiếng Phần Lan cổ, ban đầu có nghĩa là sable, sau này là cây thông. Một loài động vật săn mồi nhỏ, có bộ lông đen này từng được tìm thấy trong khu vực, nhưng hiện đã tuyệt chủng.
4) Tại Châu Á, chữ số 4 không bao giờ xuất hiện trong bất kỳ kiểu máy điện thoại Nokia nào, vì số 4 được coi là không may mắn ở nhiều nơi tại Châu Á.
5) Các điện thoại Nokia trước đây không tự động hẹn giờ cuộc gọi khi cuộc gọi được kết nối, nhưng sẽ khởi động khi cuộc gọi bắt đầu. (Ngoại trừ các thiết bị cầm tay của Series 60 như Nokia 6600 trở đi)
6) Nhạc chuông “Nokia tune” thực chất được lấy từ tác phẩm guitar nổi tiếng tại thế kỷ 19 tên là “Gran Vals” của nhạc sĩ người Tây Ban Nha Francisco Tárrega. Nokia Tune ban đầu được đặt tên là "Grande Valse” trên điện thoại Nokia nhưng đã được đổi thành “Nokia Tune” vào năm 1998 khi nó trở nên nổi tiếng đến mức mọi người gọi nó là “Nokia Tune”.
7) Cư dân của các quốc gia vùng Vịnh hơi khó nhớ số model của điện thoại Nokia. Thay vào đó, họ thích gọi cụ thể bằng tên. Nokia 6600 được biết đến nhiều hơn với tên 'Panda', 6630 với tên 'Faris', 6680 với tên 'Shaitan', v.v.
8) Kể từ khi sản xuất mẫu điện thoại đầu tiên vào năm 1982, cho đến nay Nokia đã tung ra hơn 500 mẫu điện thoại di động, cao nhất so với bất kỳ nhà sản xuất thiết bị di động nào.
9) Hai mẫu đen trắng phổ biến nhất Nokia 3310 và Nokia 3330 đã bán được khoảng 126 triệu chiếc, với 3310 là điện thoại di động đầu tiên của Nokia không có ăng-ten ngoài.
10) Nokia từng sản xuất khoảng 210 triệu thiết bị cầm tay mỗi năm, nếu tính ra thì có đến 6,5 điện thoại được sản xuất mỗi giây. Con số này hiện đã giảm xuống rất nhiều do nhu cầu giảm và cạnh tranh khốc liệt trên thị trường di động.
Trên đây là bộ hình nền điện thoại nokia 1280 cho iPhone. Bạn có thể để làm màn hình khóa trên smartphone của mình. Nếu bạn muốn Admin Quản Trị Mạng thiết kế thêm hình nền nokia 1280 với những chủ đề nào thì hãy để lại bình luận ở dưới bài viết này nhé.
Ngoài bộ hình nền 1280 cho iPhone trên đây, bạn sẽ có một bộ hình nền đặc biệt không kém đó chính là bộ hình nền trong suốt iPhone. Với bộ hình nền trong suốt này, bạn sẽ nhìn rõ linh kiện của chiếc iPhone của bạn có những gì.
Xem thêm:
- Hình nền Free Fire cho máy tính và điện thoại mới nhất
- Tổng hợp hình nền PowerPoint cảm ơn đẹp, chuyên nghiệp
- Mời tải bộ hình nền chất lượng cao của iPhone SE 2020
