
Loạt cảnh buồn nhất mọi thời đại ở hoạt hình Nhật Bản: Chi tiết của Doraemon không ai muốn xảy ra!
Đây là những tình tiết khiến khán giả rơi nước mắt ở hoạt hình Nhật Bản.
Ai bảo hoạt hình không thể khiến người xem rơi nước mắt? Hoạt hình Nhật Bản (anime) có không ít những tình tiết đau thương, để lại trong lòng khán giả sự ám ảnh khôn nguôi. Sau đây là những cảnh phim buồn và không thể nào quên nhất.
Cái chết của Setsuko
Mộ Đom Đóm cho đến nay vẫn là bộ phim buồn nhất mà Ghibli từng sản xuất, với câu chuyện xoay quanh hành trình sinh tồn của 2 anh em giữa lúc khắc nghiệt nhất. Đặc biệt, sự ngây thơ đến đáng thương của cô bé Setsuko khiến bất kì ai khi xem cũng không cầm được nước mắt. Đối mặt với đói nghèo, không nhà không cửa, chuyện gì đến cũng đã đến. Một ngày nọ khi Seita trở về, Setsuko đã nằm đó hấp hối. Một phần do quá đói, một phần nữa vì cô bé đã nuốt những chiếc cúc áo (do lầm tưởng đó là kẹo). Sau cùng, Setsuko ra đi trong vòng tay của anh trai, hi vọng ở một chân trời mới cô bé sẽ được hưởng sự ấm no và hạnh phúc.

Gin biến mất
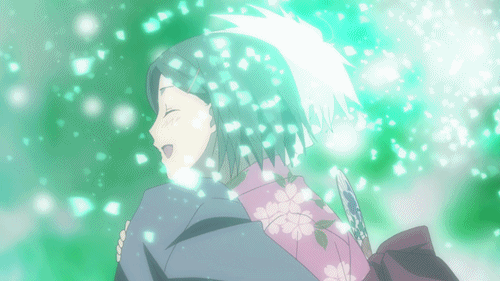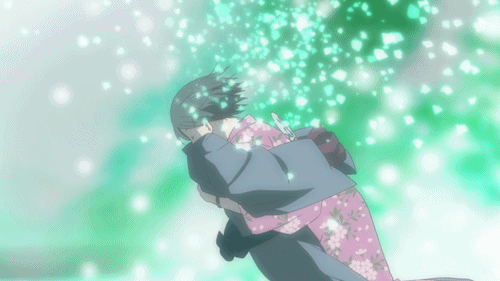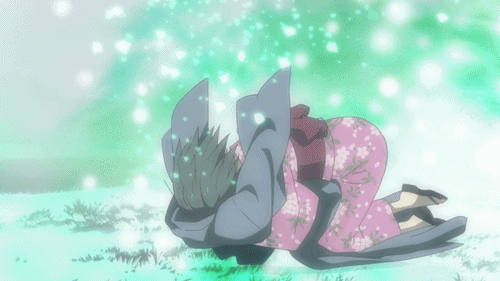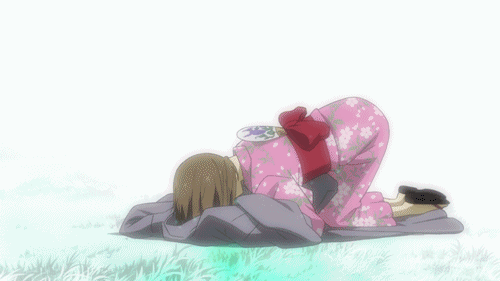
Lại một thước phim buồn có đom đóm, và lần này là Lạc Vào Khu Rừng Đom Đóm. Bộ phim kể về mối lương duyên giữa cô bé con người tên Hotaru và thần rừng Gin. Gin luôn xuất hiện với hình hài một chàng trai trẻ đeo mặt nạ, và không cho bất kì ai chạm vào mình. Đó là một lời nguyền, khi cậu và con người nào đó chạm vào nhau thì cậu sẽ lập tức biến mất. Dù rất yêu nhau nhưng Hotaru và Gin không thể có những cử chỉ thân mật. Cho đến khi Gin vô tình cứu lấy một cậu bé, lời nguyền đã phát huy khiến Gin tan biến. Khoảnh khắc Hotaru lao đến ôm cậu lần cuối chắc chắn đã khiến nhiều khán giả phải sụt sùi.
Màn hi sinh của Ace
Là một fan cứng của One Piece, chắc chắn không ai là không hiểu rõ tình anh em hơn cả trời rộng, biển sâu của Ace và Luffy. Đó là lý do mà khi Ace hi sinh, nó đã khiến hàng vạn trái tim khán giả tan vỡ, và ngay cả Luffy cũng vậy. Vì yêu thương em, Ace đã dũng cảm đứng ra lãnh đòn chí mạng, khiến anh lập tức lịm đi. Luffy chỉ có thể chứng kiến anh mình mang cả tính mạng ra để bảo vệ mình, rồi không bao giờ tỉnh lại nữa. Cho đến hiện tại, đây vẫn là khoảnh khắc buồn bậc nhất trong lịch sử hoạt hình Nhật Bản.

Sasha bị bắn
Attack on Titan mở ra một thế giới đầy hấp dẫn và lý thú nhưng không kém phần sâu sắc, trong đó con người phải chiến đấu để lật đổ các Titan khổng lồ và những bí mật theo sau chúng. Cùng nhau tập luyện để trở thành những chiến binh xuất sắc, Eren và nhóm bạn của mình đã sốc đến cỡ nào khi thành viên Sasha bất ngờ qua đời. Khi tấn công Liberio, hạm đội của Eren không ngờ rằng cô bé quả cảm Gabi đã lẻn lên tàu, sau đó bắn Sasha. Khi nằm xuống, Sasha đã nói lời cuối "Thịt", tức món ăn mà cô luôn ao ước được ăn khi hòa bình đến. Điều này đã khiến ai nấy đau khổ, còn Eren thì vừa cười vừa khóc trong đau đớn.

Doraemon trở về tương lai
Là chú mèo máy được gửi đến hiện tại để giúp Nobita trưởng thành, tất nhiên rồi đến thời điểm nào đó thì cả hai phải nói lời tạm biệt. Từng có một tập phim khi Doraemon quyết định đã đến lúc mình phải đi, sau đó lén trở về tương lai và không trở lại. Tuy nhiên Doraemon có để lại cho Nobita một món bảo bối phòng thân - Thuốc nói dối. Sau cùng, Nobita đã dùng bảo bối này để mang Doraemon trở về, bằng cách nói rằng "Doraemon sẽ không bao giờ trở về". Tuy có đoạn kết hạnh phúc nhưng chắc chắn việc Doraemon - Nobita chia xa là "cơn ác mộng" mà không khán giả nào muốn xảy đến.

Thầy Koro ra đi
Đây đích thị là khoảnh khắc buồn nhất được nhiều khán giả bình chọn trên trang Ranker nổi tiếng. Đến với Trái Đất với mục đích hủy diệt, Korosensei đã trở thành giáo viên của lớp 3-E và hi vọng sẽ có ai đó đủ sức để ngăn hắn lại. Thế nhưng dần dà, chính Korosensei đã trở thành nguồn động lực giúp các học sinh lớp 3-E trưởng thành, trở nên giỏi giang. Thế nhưng rồi Trái Đất vẫn phải có ai đó cứu giúp. Khoảnh khắc Korosensei chấp nhận cho Nagisa tiêu diệt mình đã khiến các học sinh và ngay cả khán giả phải khóc nức nở.

Nguồn ảnh: CrunchyRoll
Admin